More Information
Submitted: 30 March 2020 | Approved: 13 April 2020 | Published: 14 April 2020
How to cite this article: Corona A, Persico P, Vercelli A, Gramenzi A, Cornegliani L. In vitro antimicrobial activity of a black currant oil based shampoo versus a chlorhexidine 4% shampoo on bacteria strains isolated from canine pyoderma: A comparative study. Insights Vet Sci. 2020; 4: 014-017.
DOI: 10.29328/journal.ivs.1001021
Copyright License: © 2020 Corona A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In vitro antimicrobial activity of a black currant oil based shampoo versus a chlorhexidine 4% shampoo on bacteria strains isolated from canine pyoderma: A comparative study
Antonio Corona1*, Paola Persico2, Antonella Vercelli1, Alessandro Gramenzi3 and Luisa Cornegliani1
1“Città di Torino” Veterinary Clinic, c.so Traiano 99/c, 10135 Turin, Italy
2Freelance Veterinarian, Milan, Italy
3Faculty of Veterinary Medicine, Teramo, Italy
*Address for Correspondence: Antonio Corona, “Città di Torino” Veterinary Clinic, c.so Traiano 99/c, 10135 Turin, Italy, Email: [email protected]
Over the last few years, antimicrobial shampoo therapy has been increasingly used to treat skin infections in order to reduce systemic use of antibiotics. This study was aimed to compare the In vitro bactericidal effect of a black currant oil based shampoo (S1) to a chlorhexidine 4% shampoo (S2) against methicillin-sensitive Staphylococcus pseudintermedius (MSSP), methicillin-resistant Staphylococcus pseudintermedius (MRSP), Staphylococcus aureus (SA), Escherichia coli (EC) and Pseudomonas aeruginosa (PA) isolates.
A collection of 50 bacterial strains from skin swabs of dogs with superficial recurrent pyoderma was selected: 10 MSSP, 10 MRSP, 10 SA, 10 EC and 10 PA. The two shampoos were blindly tested in duplicate with a microdilution plate method, with scalar concentrations from 1:2 to 1: 256. The MBC was performed for each dilution. A linear regression was used to detect a statistically significance between the two shampoos.
All isolates were completely killed at 1:2 up to 1:16 dilution of the two antiseptic products. At the 1:32 dilution the first bacterial growths were observed, in particular for 2 and 4 strains of MRSP by S1 and S2 respectively. The first lethal dilution for SA was at 1:64 for S1/S2 and only for S2 against SP. No significant difference was observed between the two shampoos according to the results of linear regression significant for: i) MRSP, PA and EC (p < 0.05); ii) MSSP and SA (p < 0.1).
This study showed that both black currant oil based shampoo and chlorhexidine 4% shampoo have a similar In vitro bactericidal activity.
Over the last decade, antimicrobial shampoo therapy has been increasingly used to treat skin infections [1,2]. This new approach is related to the increasing occurrence of antibiotic resistance in veterinary and human medicine. Thus, new strategies and topical treatments are required to address this issue[1-5]. Creams or ointments containing topical antibiotics may be applied on the skin as well as shampoos with antiseptics (e.g. chlorhexidine 2%-4%, benzoyl peroxide and ethyl lactate [6]). International guidelines suggest the use of topical antimicrobial shampoos and sprays for mild superficial, surface and/or focal infections [7,8]. This approach is particularly important if referred to multi-antibiotic resistant bacteria such as methicillin-resistant Staphylococcus pseudintermedius (MRSP) and Pseudomonas aeruginosa (PA), as these two bacteria can be difficult to manage using systemic antibiotics alone [9,10]. In fact, these bacteria may be resistant to all oral antibiotics, so the use of topical antimicrobial products, including antiseptics, is recommended to treat surface skin infections [11]. The active ingredients of topical treatments act primarily on the site of infection and the antimicrobial concentrations obtained may be more effective against resistant strains. Among antiseptics used in topic therapy benzoyl peroxide is reported to be an effective topical antimicrobial [12,13] and chlorhexidine is effective against staphylococci, Pseudomonas and Malassezia [14,15]. Black currant seed oil is derived from the seeds of Ribes nigrum and recently it has been given some attention as a source of PUFAs (Polyunsaturated Fatty Acids), in humans and dogs with AD [16]. It contains an unusually high amount of omega-3 and -6, linoleic and stearidonic acid, flavonoids, phenolic acids and proanthocyanidins. For these reasons it should be preferred in the treatment of inflammatory skin diseases owing to its physiological antiallergic, anticarcinogenic, antihypertensive, antiarthritic and antimicrobial activities [17].
This study was aimed to compare the In vitro bactericidal effect of a black currant oil based shampoo (S1) to a chlorhexidine 4% shampoo (S2) against methicillin-sensitive S. pseudintermedius (MSSP), methicillin-resistant S. pseudintermedius (MRSP), S. aureus (SA), Escherichia coli (EC) and P. aeruginosa (PA) isolates.
Bacteria isolates and media
A collection of 50 bacteria strains obtained from skin swabs of different dog breeds with superficial recurrent pyoderma was selected. The bacteria strains were represented by 10 MSSP, 10 MRSP, 10 SA, 10 EC and 10 PA. The swabs were inoculated onto Tryptone Soya Agar plates with 5% sheep’s blood, Mannitol Salt Agar and McConkey Agar (Oxoid®, Italy), incubated at 37 °C for 24h, before the final reading. Based on phenotypic identification standards, pure isolates were subjected to Gram staining, catalase and oxidase test. Biochemical-enzymatic identification was performed by API® galleries with apiweb database (bioMérieux®, France) and with an acceptable value ≥ 85% for species affinity. The staphylococci that were identified by the system as Staphylococcus intermedius are here referred to as S. pseudintermedius (SP) based on recent classifications of the related group [18].
To check methicillin-resistant strains Kirby-Bauer oxacillin diffusion test (ODD - 5 µg/mL) was performed, in accordance with the indications of the Clinical Laboratory and Standard Institute [19] and onto selective Brilliance MRSA2 Agar [20] (Oxoid®, Italy). Out of the 50 bacterial strains, 10 are MRSPs, 20 are MSSPs and MSSAs, and 20 bacterial strains (EC and PA) are non-MDR (Multi-Drug Resistant [21]) population.
In vitro susceptibility test
The two biocide shampoo products used in this study (S1 and S2) are shown in table 1, along with their active ingredients. The two shampoos were blindly tested in duplicate with a microdilution plate method [22,23]. The bacterial strains were isolated in pure culture and suspended in saline phosphate buffer (PBS; pH 7.2) up to turbidity equal to 0.5 McFarland, by means of densimeter (DEN-1B, Biosan® Sia, Latvia) and corresponding to a concentration of approximately 108 CFU/mL of bacteria. The bacterial suspension was then further diluted 1:100 and to obtain a final concentration of 106 CFU/mL it was used for serial dilutions in a 96-well sterile cell culture plate containing 100 µl of the shampoo to be tested, with scalar concentrations from 1:2 to 1: 256. Then, 100 µL of PBS without antiseptic were added to the bacterial suspension and served as positive control (PC), while shampoo alone was used as negative control (NC). After 30 min incubation at + 37 °C, an aliquot of 10 µl was taken from each well and plated onto Tryptone Soya Agar with 5% sheep’s blood and finally incubated for a further 24 hours. The count of bacterial colony forming units (CFU) was therefore visually performed on a plate up to a maximum of 100 and then defined as “confluent” when the units were too numerous to be counted individually. The minimum bactericidal concentration [24] (MBC) was defined as the lowest concentration capable of inhibiting bacterial growth of at least 99.9% of the initial population [25] (absence of growth on plate) and was therefore evaluated.
| Table 1: strains growth at progressive dilutions and corresponding CFU. Shampoos used in the study. | ||||
| pH as | ||||
| Shampoo | Ingredients | Manufacturer’s pH | used | |
| S1 | Ribes Pet Ultra shampoo® (NBF Lines®, Milan, Italy) |
Blackcurrant seed oil, ac.18β-glycirretic, octopirox, vegetable protein, Vit.E, selected ceramide complex, menthol | 6,5 | 6,5 |
| S2 | Clorexyderm® solution 4% (Icf, Cremona, Italy), | Chlorhexidine digluconate 4%, Propylene glycol; Glycerin; Lanolin; Fatty alcohol ethoxylate |
n.d. | n.d. |
Statistical analysis
A linear regression was used to detect statistically significance between the two shampoos tested, considering the number of bacterial colonies and shampoo dilutions.
In all cases, the microbial isolates incubated in PC line resulted in confluent growth. No growth was seen in the NC wells containing shampoos alone.
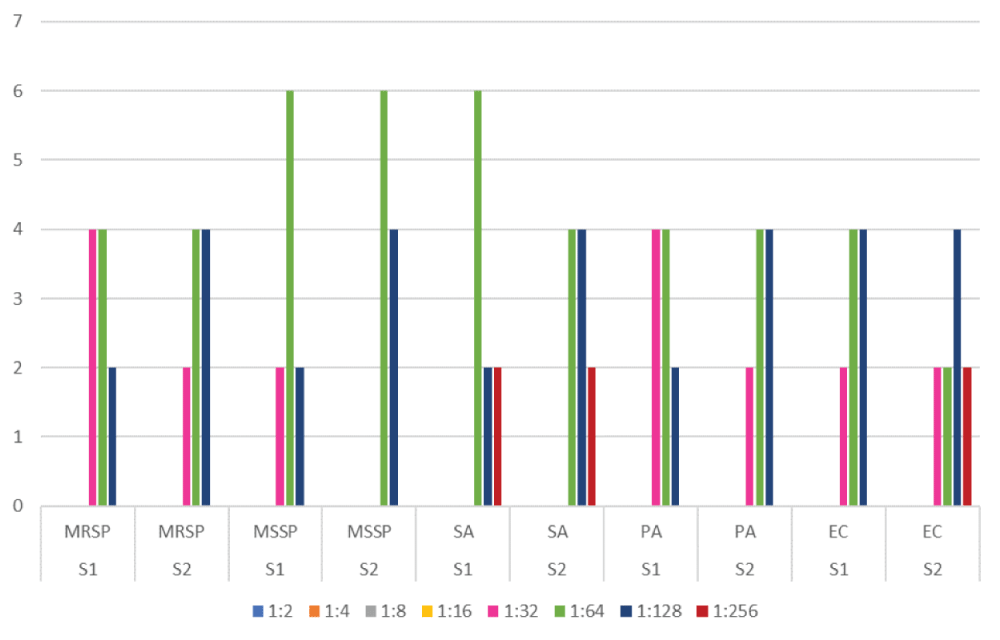
The products showed bactericidal activity against all pathogens tested as shown in figure 1. MBCs differed substantially depending on the species of microorganism but usually varied by two-fourfold dilutions only among strains belonging to the same species. All isolates were completely killed at 1:2 up to 1:16 dilution of the antiseptic product (Table 2). At the 1:32 dilution the first bacterial growths were observed, in particular for 2 and 4 strains of MRSP by S1 and S2 respectively. The first lethal dilution for SA was at 1:64 for S1/S2 and only for S2 against SP.
| Table2: strains growth at progressive dilutionsand corresponding CFU. | |||||||||||
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | ||
| MRSp | MRSp | MSSp | MSSp | SA | SA | PA | PA | EC | EC | CFU/ml | |
| 1:2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1:4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1:8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1:16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1:32 | 4 | 2 | 2 | 0 | 0 | 0 | 4 | 2 | 2 | 2 | 0-10 |
| 1:64 | 4 | 4 | 6 | 6 | 6 | 4 | 4 | 4 | 4 | 2 | 11-20 |
| 1:128 | 2 | 4 | 2 | 4 | 2 | 4 | 2 | 4 | 4 | 4 | 21-50 |
| 1:256 | 2 | 2 | 2 | >100 | |||||||
Figure 1: The products showed bactericidal activity against all pathogens tested.
The CFU detected were homogeneous among the different bacterial species and in relation to the dilutions performed it was possible to identify progressive growth ranges up to the 1: 256 dilution with confluent appearance (Table 2).
No significant difference was observed between two shampoos. Only the dilution used was significant for the bacterial growth proliferation for MRSP, PA and EC (p < 0.05), MSSP and SA (p < 0.1).
The black currant oil based shampoo turned out to have an active In vitro efficacy against the most common skin bacteria responsible for canine pyoderma. Furthermore, this study revealed that both black currant oil based shampoo and chlorhexidine 4% shampoo have a similar In vitro bactericidal activity. Previous research on similar formulations showed In vitro antimicrobial characteristics of products for skin use or otology [22-26]. These studies described the bactericidal capacity of known local antiseptics such as gluconate chlorhexidine in different concentrations, Tris-EDTA, isopropyl alcohol, benzoyl peroxide, often associated with low pH values of the disinfectant solution or other components with antimicrobial activity. Chlorhexidine 4% (S2), which can display mechanisms of resistance against Gram-negative bacteria and S. aureus [27], here showed killing activity at 1:32 and at 1:64 respectively. The latter dilution (1:64) is the same also for MSSP while S1 shows bactericidal activity at 1:64 only for SA. In all other cases S1 is active up to 1:32. Otherwise the two shampoos differ only in the number of strains killed at the same dilution (Table 2). The black currant oil based shampoo (S1) tested in this study has shown antibacterial and anti-inflammatory activities as well as skin protection and soothing effects, as it claims. However, the real bactericidal efficacy against the most common bacteria responsible for canine superficial pyoderma had never tested In vitro until now. Over the past decade, there has been an increasing interest in the use of topical natural products with antimicrobial activity both as a single or an adjuvant therapy for the treatment of superficial bacterial or fungal infections in dogs [4,28,29] . With regards to S1 it is possible to assert that black currant seed oil, as other studies have revealed, displays an In vitro antimicrobial activity [30,31]. Unfortunately, it was not possible to evaluate which is the component or which are the components with the specific antibacterial activity. Among the S1 molecules, the antiseptic action of pyroctolamine, thanks to its intrinsic broad-spectrum antibacterial activity towards Gram positive, Gram negative and mycotic germs [32], is well known. The 18 β-glycyrrhetinic acid, vegetable proteins, tocopherol and a complex of ceramides have instead an emollient and protective action against the skin barrier. No antibacterial activity has ever been described for these components. Thus, the authors hypothesize that it is the combination of the components which exhibits an In vitro antibacterial action capable of determining bacterial death within 30 minutes of its contact at dilutions four times higher than the starting product.
This study has confirmed how the black currant seed oil based shampoo can be considered an antiseptic with a local action. Furthermore, this product has an antibacterial In vitro activity comparable to that of chlorhexidine 4% [23,30] against the most common bacteria responsible for canine pyoderma. Further studies are however required to test the in vivo efficacy of this shampoo in controlling superficial skin infections.
The authors thank Dr Carlo Maria Colombo and Giuliano Pappini for NBF partial financial support, Dr Daniele Di Simone for statistical support (Institute of Clinical Physiology, CNR), the Laboratory Technical Staff of Città di Torino Veterinary Clinic is also gratefully acknowledged.
Source of funding
This study was partially supported by NBF Lanes Italy.
Conflict of interest
One of the authors is an NBF Lanes Consultant.
- Rosenkrantz W. Practical applications of topical therapy for allergic, infectious, and seborrheic disorders. Clin Tech Small Anim Pract. 2006; 21: 106–116.PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16933477
- Guaguere E. Topical treatment of canine and feline pyoderma. Vet Dermatol. 1996; 7: 145–51.
- Layne EA. Can pyoderma in dogs be treated with fewer antibiotics? Vet Rec. 2019; 184: 736-738. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31197051
- Bajwa J. Canine superficial pyoderma and therapeutic considerations. Can Vet J. 2016; 57: 204-206. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4713004/
- Papadogiannakis E, Velonakis E, Vatopoulos A. Ever-increasing emergence of multi-drug resistant Staphylococcus pseudintermedius in the dog and its zoonotic potentials. J Hellenic Vet Medical Soc. 2018; 67: 109-116.
- Miller WH, Griffin CE, Campbell KL. Dermatologic Therapy. In: Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis: Elsevier, 2013; 108–183.
- Beco L, Guaguère E, Lorente Méndez C, Noli C, Nuttall T, et al. Suggested guidelines for using systemic antimicrobials in bacterial skin infections: part 2 antimicrobial choice, treatment regimens and compliance. Vet Rec. 2013; 19: 72–78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23292948
- Hillier A, Lloyd DH, Weese JS, Blondeau JM, Boothe D, et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal infectious Dis- eases). Vet Dermatol. 2014; 25: 163–175. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24720433
- Hillier A, Alcorn JR, Cole LK, Kowalski JJ. Pyoderma caused by Pseudomonas aeruginosa infection in dogs: 20 cases. Vet Dermatol. 2006; 17: 432–439. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17083575
- Loeffler A, Linek M, Moodley A, Guardabassi L, Sung JM, et al. First report of multiresistant, mecA-positive Staphylococcus intermedius in Europe: 12 cases from a veterinary dermatology referral clinic in Germany. Vet Dermatol. 2007; 18: 412–421. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17991158
- Guardabassi L, Houser GA, Frank LA. Guidelines for antimicrobial use in small animals. In: Guardabassi L, Jensen LB, Hilde K. eds. Guide to Antimicrobial Use in Animals. Oxford: Blackwell Pub. 2008: 183–206.
- Scott DW. Clinical assessment of topical benzoyl peroxide in treatment of canine skin diseases. Vet Med Small Anim Clin. 1979; 74: 808–813. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/256688
- Scott DW, Miller WH, Cayatte SM. A clinical study on the effect of two commercial veterinary benzoyl peroxide shampoos in dogs. Canine Practice 1994; 19: 7–10.
- Bond R, Rose JF, Ellis JW, Lloyd DH. Comparison of two shampoos for treatment of Malassezia pachydermatis associated seborrhoeic dermatitis in basset hounds. J Small Anim Pract. 1995; 36: 99–104. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7783442
- Lloyd DH, Lamport AI. Activity of chlorhexidine shampoos In vitro against Staphylococcus intermedius, Pseudomonas aeruginosa and Malassezia pachydermatis. Vet Rec 1999; 144: 536–537. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10378283
- Noli C, Carta G, Cordeddu L, Melis MP, Murru E, et al. Conjugated linoleic acid and black currant seed oil in the treatment of canine atopic dermatitis: a preliminary report. Vet J. 2007; 173: 413-421. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16495095
- Marchegiani A, Fruganti A, Spaterna A, Dalle Vedove E, Bachetti B, et al. Impact of Nutritional Supplementation on Canine Dermatological Disorders. Vet Sci. 2020; 7: E38. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/32260299
- Devriese LA, Hermans K, Baele M, Haesebrouck F. Staphylococcus pseudintermedius versus Staphylococcus intermedius. Vet Microbiol. 2009; 133: 206-207. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18760884
- Clinical Laboratory and Standard Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standards – 4th edition. CLSI document VET01-A4. Wayne, PA: CLSI; 2013.
- Horstmann C, Mueller RS, Straubinger RK, Werckenthin C. Detection of methicillin-resistant Staphylococcus pseudintermedius with commercially available selective media. Lett Appl Microbiol. 2012; 54: 26-31. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22023239
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18: 268-281. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21793988
- Guardabassi L, Ghibaudo G, Damborg P. In vitro antimicrobial activity of a commercial ear antiseptic containing chlorhexidine and Tris–EDTA. Vet Dermatol. 2009; 21: 282-286. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20030799
- Swinney A1, Fazakerley J, McEwan N, Nuttall T. Comparative In vitro antimicrobial efficacy of commercial ear cleaners. Vet Dermatol. 2008; 19: 373–379. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19055612
- CLSI, Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline, CLSI document M26-A. Clinical and Laboratory Standards Institute, 950 West Valley Roadn Suite 2500, Wayne, Pennsylvania 19087, USA, 1998.
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for In vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016; 6: 71-79. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29403965
- Swinney A, Fazakerley J, McEwan N et al. Comparative In vitro antimicrobial efficacy of commercial ear cleaners. Vet Dermatol 2008; 19: 373–379. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19055612
- Lloyd DH, Lamport AI. Activity of chlorhexidine shampoos In vitro against Staphylococcus intermedius, Pseudomonas aeruginosa and Malassezia pachydermatis. Vet Rec. 1999; 144: 536–537. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10378283
- Loeffer A, Lloyd DH. What has changed in canine pyoderma? A narrative review. Vet J. 2018; 235: 73-82. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29704943
- Oliveira AMP, Devesa JSP, Hill PB. In vitro efficacy of a honey‐based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet Dermatol. 2018; 29: 180-e65. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29569291
- Widén C, Renvert S, Persson GR. Antibacterial activity of berry juices, an In vitro study. Acta Odontol Scand. 2015; 73: 539-543. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25727734
- Miladinović B, Kostić M, Šavikin K, Đorđević B, Mihajilov-Krstev T, et al. Chemical profile and antioxidative and antimicrobial activity of juices and extracts of 4 black currants varieties (Ribes nigrum L). J Food Sci. 2014; 79: 2014. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24506271
- Bourdeau P, Blumstein P, Marchand AM, Gardeyc L, Jasmin P, et al. An in vivo procedure to evaluate antifungals agents on Malassezia pachydermatis in dogs: example with a piroctone olamine containing shampoo. J de Mycologie Médicale. 2006; 16: 9-15.