More Information
Submitted: 26 June 2020 | Approved: 24 July 2020 | Published: 27 July 2020
How to cite this article: Fesseha H, Negash G. Evaluation of Single Bilateral Intratesticular Injection of Cetrimide for Nonsurgical Sterilization of Adult Male Albino Mice. Insights Vet Sci. 2020; 4: 025-034.
DOI: 10.29328/journal.ivs.1001023
ORCiD ID: orcid.org/0000-0001-6516-3036
Copyright License: © 2020 Fesseha H, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Albino mice; Cetrimide; Histopathology; Testicle; Chemical sterilization; Intratesticular injection
Evaluation of Single Bilateral Intratesticular Injection of Cetrimide for Nonsurgical Sterilization of Adult Male Albino Mice
Haben Fesseha1* and Guesh Negash2
1School of Veterinary Medicine, Wolaita Sodo University, PO. Box 138, Wolaita Sodo, Ethiopia
2College of Veterinary Science, Mekelle University, PO. Box 2084, Mekelle, Ethiopia
*Address for Correspondence: Haben Fesseha, Department of Veterinary Surgery and Diagnostic Imaging, School of Veterinary Medicine, Wolaita Sodo University, PO. Box 138, Wolaita Sodo, Ethiopia, Tel. +251910737790; Fax: +251465515113; Email: [email protected]
Nonsurgical fertility control is increasingly advocated as more cost-effective than surgical sterilization to manage stray animal populations in a different part of the world. An experimental study was conducted from December 2018 to April 2019 at Mekelle University to evaluate the effect of single bilateral intratesticular injection of cetrimide 2% in adult albino mice. A total of 20 clinically healthy albino mice selected based on their age and sex and were divided randomly into five groups and evaluation was conducted for 30 days after intratesticular injection of cetrimide solution 2% at the dose rate of 5, 10, 15 and 20 mg per testis and for control 0.1 mL normal saline per testis per 100 g body weight were given. All albino mice were evaluated for 30 days at a fixed interval. Change in body weight, scrotal width, sexual behavior, and fertility performance was also assessed. On day 30, all albino mice were sacrificed for histopathological study. Means ± Standard deviation of the mean, one-way, and a mixed model ANOVA (for repeated measures) was used to summarize the data, determine the effects of group and time on bodyweight and scrotal width. The significant increase in body weight (p - 0.001) and significant reduction of scrotal width (p - 0.001) were noted in all cetrimide treated in comparison to control groups. In addition, there was a significant (p < 0.05) reduction of scrotal width in albino mice after intratesticular injection of cetrimide on day 1, 10, 15, 20, 25, and 30 with respect to their experimental groups. Testicular histology revealed that there were multinucleated giant cells in seminiferous tubules, derangement of tubular architecture along with infiltration of leucocytes and appearance of fibrous tissue were seen on testicular sections at a dose rate of 15 and 20 mg. Similarly, a significant change in the sexual behavior of the treated males and no pregnancy was detected on female albino mice after 21 days post-coital at 10, 15, or 20 mg cetrimide-treated males. In conclusion, a single bilateral intratesticular injection of cetrimide 2% at a dose of 15 and 20 mg might provide an effective way of sterilization and may be considered as an alternative to surgical castration in male animals. Besides, further assessment should be done in the future to identify the mechanism of infertility.
Castration is one of the most common surgical procedures and is usually performed to sterilize animals which are unsuitable for the genetic pool and also to eliminate masculine behavior as well as reduce public nuisance [1]. Veterinarians are still practicing the open surgical method of castration which is the most effective and the only means of sterilization for male animals. Yet, castration by open surgery requires postoperative care to minimize the risk of hemorrhage and infection. Besides, this method has some disadvantages: it is not cost-effective and time-consuming with the risk of severe post-surgical complications [2-4].
In contrast to the surgical method, the challenge has been taken up by different reproductive biologists to develop a method of chemical sterilization, which may be a better alternative to surgical castration, as well as suited for mass-scale sterilization of male domestic animals without postoperative hazards [2,5]. Besides, over the last decades, different chemical agents were tried to bring about castration using inorganic chemicals, immunocontraceptives, and hormones including androgen [6], progestogens [7], androgens plus progestagens [8,9] and agonists for Gonadotrophin Releasing Hormone (GnRH) [10].
Different researchers have evaluated nonsurgical sterilization with the injection of various hormones in many species of male animals, but these treatments failed to induce permanent sterility. Immunization techniques have also been used to induce antibodies against gonadotrophins and GnRH and had indicated that such immunization techniques vary in the effectiveness and duration of azoospermia. Adverse vaccination reactions were also observed as another disadvantage [11,12].
Accordingly, several attempts have been made in search of promising effective chemical agent, which included intratesticular injections (ITIs) of various chemical agents to promote castration in various species such as Rhesus monkey (iron salt) [13], Mice (Chlorhexidine) [4,14], Rats (Hypertonic saline solution, and Calcium chloride) [15-18], Canine (Calcium chloride, Zinc gluconate, Ethanol, Eugenia caryophyllata essential oil and Glycerol) [5,12,19-23], Feline (Calcium chloride, Zinc gluconate) [24-26], Bovine (hypertonic sodium chloride) [27-29], Donkey (Calcium chloride) [2], Ovine (Formalin) [30] and Caprine (Calcium chloride and chlorhexidine gluconate & cetrimide) [31,32].
Chemical sterilization has found application in some species of male animals such as monkeys, goats, bulls, hamsters, rabbits, and dogs [28, 31]. Hence, researchers over the past years have also tried various chemical agents such as Danazol, glycerol, Lactic acid, Ferric chloride, and Ferrous sulfate, Calcium chloride (CaCl2), Calmette-Gue´rin (BCG), Zinc gluconate (Neutersol) and 20% hypertonic saline solution for induction of chemosterilization [33]. However, all these chemical agents, following intratesticular injection had exhibited pain, pyrexia, and even severe testicular inflammation (orchitis). Some agents like, cadmium chloride, glycerol, lactic acid had caused selective destruction of testicular tissue [21,34] with reversible testicular tissue damage [35].
Even though chemicals used had an effect on the destruction of testicular tissue, it had also complications and some drawbacks. For instance, in some cases, the interstitial portion of seminiferous tubules had regenerated after an initial phase of testicular atrophy and this had led to secondary male behavior causing management problems of the animals [36]. Due to such types of complications caused by the use of the aforementioned chemicals, an effective chemosterilizing agent is yet to be established.
Similarly, different researches are still being conducted and researchers have been interested in developing a method for chemical castration which might be a better alternative to the surgical method [33,37]. Moreover, the surgical method is not effective for large-scale applications, especially for controlling the large population size of undesirable mammals in the community like stray dogs. Besides this, postoperative care and management of the animal were also required to prevent infection [31]. Besides, vasectomies and vassal occlusion are less invasive surgical procedures than castration, still, these procedures also carry similar anesthetic risks and postsurgical complications [2,3].
An ideal chemical sterilizing agent should be permanent, low-cost treatment, effectively arrests spermatogenesis and androgenesis and not affecting the welfare of animals as well as without side effects [4,5,33,38]. Thus, the objective of this current experiment was to determine the effective dose and evaluate the efficacy of cetrimide 2% injection bilaterally in testicular tissues for induction of chemosterilization in albino rats based on histological examination of testicular tissue and based on the libido and fertility performance of the male albino mice.
Study animals
Twenty adult male albino mice of Swiss strain, 90 days of age, weighing between 39-52 gm, were selected and housed four albino mice per cage at ambient temperature (22 ± 2 0C) and humidity (60 ± 5%) in a photoperiod-controlled room (light/dark:14:10 hr) with free access to standard laboratory food and water ad libitum. The mice were allowed to acclimatize to the laboratory conditions by keeping them for 15 days ahead of the experimentation.
The National Institute of Health, Guide for the Care and Use of Laboratory Animals [39] was followed throughout the experimental duration. The experimental protocol also met the Guidelines for Care and Use of Animals in Scientific Research [40] and all procedures of the current work were duly approved by the Animal Ethics and Experimentation Committee (AEEC) of the College of Veterinary Science, Mekelle University.
Experimental design
The experimental study design was conducted from December 2018 to April 2019 using clinically healthy male albino mice to evaluate the effect of 2% Cetrimide after a single bilateral intratesticular injection. In order to determine the efficacy of a single intratesticular injection of Cetrimide for chemosterilization, twenty mice were treated with sterile analytical grade Cetrimide (Cetrimide; Sheba pharmaceuticals, Addis Ababa, Ethiopia).
Accordingly, all experimental male albino mice (n = 20) were divided into five groups. Four clinically healthy male albino mice were grouped to each experimental group by a complete randomized blocking technique based on body weight and age [41]. The groups were then randomly allocated to five different groups according to dose levels of Cetrimide. All the five groups were kept in a separate cage in the pathology laboratory research unit of College of Veterinary Science, Mekelle University. The body weight and scrotal width record of each experimental male albino mice were collected from the starting day of the experiment (pre-treatment) until the end of the experiment (day 30 post-treatment).
Administration of cetrimide solutions
All experimental albino mice were allocated into five groups and were kept in a separate cage. Besides, all groups were kept under similar environmental conditions and provided with the same ad libitum food and water except for the different doses of treatment as listed below.
Group 1: Four mice each of them were given a single bilateral intratesticular injection of sterile 0.1mL normal saline per testis per 100g body weight and were considered as a control group with an average weight ranges between 44.43 ± 2.45 grams
Group 2: Four mice each of them were given a single bilateral intratesticular injection of cetrimide 2% at a dose of 5 mg per 100 g bodyweight per testis with an average weight ranges between 45.48 ± 2.28 grams.
Group 3: Four mice each of them were given a single bilateral intratesticular injection of cetrimide 2% at a dose of 10 mg per 100g bodyweight per testis with an average weight ranges between 47.68 ± 6.19 grams.
Group 4: Four mice each of them were given a single bilateral intratesticular injection of cetrimide 2% at a dose of 15 mg per 100 g bodyweight per testis with an average weight ranges between 41.35 ± 4.72 grams.
Group 5: Four mice each of them were given a single bilateral intratesticular injection of cetrimide 2% at a dose of 20 mg per 100 g bodyweight per testis with an average weight ranges between 42.03 ± 4.33 grams.
Technique of intratesticular injection of cetrimide solutions
All male albino mice were controlled under light ether anesthesia and restrained in a dorsal recumbent position and the scrotum was thoroughly scrubbed and cleaned with 70% Alcohol solution. Intratesticular injections were carefully performed. All injections were performed using a 0.5 mL 100 unit insulin syringe with a 32G, 4 mm needle, and a separate needle was used for each testis.
The injection was administered in the cranial area of the testis and the needle was directed from the caudoventral aspect of each testis approximately 0.5 cm from the epididymal tail towards the dorsocranial aspect of that testis, with the needle parallel to the long axis of the testis. The solution was carefully deposited along the entire axis of the testis by linear infiltration while withdrawing the needle from proximal to the distal end. Necessary care was also taken to prevent the seepage of the solution from the injection site.
Post-intervention monitoring and clinical observation in albino mice
All of the experimental albino mice were kept under clinical observation from day 0 (before the experiment) to 30 days. Changes in the behavior of albino mice (General attitude), food and water consumption, gross changes in scrotal width, and ability to walk were evaluated daily till the end of the experiment (from day 1 up to day 30).
At the same time, bodyweight and scrotal width of all experimental albino mice were measured and recorded starting from day 0 (immediately before treatment) and then after the weight and scrotal width of albino mice were measured on days 1, 5, 10, 15, 20, 25 and 30 post-experiment. Body weights were measured using weight balance and their weight was recorded in gram (gm) and besides, scrotal width was used as an index of testicular size [42]. Scrotal width of both the right and left testis were measured using laboratory calipers and were recorded in millimeter (mm). Data were expressed as a mean between the width of the left and right testicles.
Evaluation of sexual behavior and fertility performance
On the 30th day post-injection period, sexual behavior and fertility performance of both the control group and cetrimide-treated albino mice were evaluated. Thus, each individual male albino mice was allowed to mate with mature, healthy, fertile, virgin, and normal cyclical (4-day estrous cycle) female albino mice. Each male albino mice was caged with three females for six consecutive days. The sexual behavior was observed and recorded for both control male albino mice and cetrimide-treated male albino mice. Besides, the behavioral changes including noising, genital sniffing, chasing, grasping, mounting, resting, eating, drinking, and aggression were also assessed. All mated females were kept individually caged and kept for 21 days to check for their pregnancy.
Histopathological study of testes
All the albino mice were sacrificed after 30 days according to the protocol used by [16,17]. The albino mice were sacrificed under light ether anesthesia and both testes were taken from each albino mice and used for histopathological study. Testicular samples taken were sectioned transversely and examined grossly. Representative samples from gross lesions of testis were taken. Then, testicular samples from each albino mice were immersed in 10% neutral buffer formalin for 48 hours after the removal of tunica albicans to prepare the sample for further automated tissue processing.
The samples were then trimmed and prepared in 1×1cm size and dehydrated in a graded alcohol series followed by embedding in paraffin wax (melting point 55-58 oC). Paraffin blocks were prepared and serial sections from the middle part from each testis were taken at a thickness of 5 μm using a rotatory microtome. These sections were stained with hematoxylin-eosin staining and mount using DPX mountant. The qualitative study of testicular tissue including seminiferous tubules and histological architecture of seminiferous tubules, interstitial space, germ cell arrangement in tubules of each animal were undertaken and then slides were examined by light microscope [43]. Histopathological lesions of testes were scored according to [44].
Data management and statistical analysis
Data collected from the laboratory were recorded in the format developed for this purpose (Annex-I) and later on entered into Microsoft Excel 2016 and analysis was carried out using a standard statistical software program (STATA version 13). The data summarized using descriptive statistics and all values were expressed in as Means ± Standard deviation of the mean. Besides, one-way ANOVA was used to determine the effects of Cetrimide as well as to illustrate the relationships between the dependent variables (bodyweight and testicular width of Albino mice) and independent variables (Cetrimide). A mixed model ANOVA (for repeated measures) was used to determine the effects of group and time on bodyweight and scrotal width. Differences were considered statistically significant a p < 0.05. Besides, the results of the histopathological finding of each group of treatment as well as the control group were recorded and their interpretations were presented with their respective groups and figures.
Ethical considerations
Ethical clearance and approval was obtained before experimental work from the Animal Ethics and Experimentation Committee (AEEC) of the College of Veterinary Science, Mekelle University.
In this experimental study, all-male albino mice were clinically monitored and assessed post experiment for 30 days after intratesticular injection of cetrimide 2% at doses of 5, 10, 15, and 20 mg/100 g body weight. Thus, based on clinical observation, all treated albino mice tolerated the intratesticular injections of Cetrimide and did not suffer from any distress, marked inflammatory swelling of the testis. There was no apparent discomfort exhibited by any of the mice, either on recovery from ether anesthesia after the injection or at any point during the experiment.
There was no apparent change in food consumption among the five groups of animals throughout the experimental duration. None of the mice injected with Cetrimide at different doses showed signs of morbidity or mortality during the studies. Postmortem inspection of each treated animal had indicated that there was no injury in the cauda epididymis, due to intratesticular injection of this agent. The effect of single bilateral intratesticular injection of cetrimide 2% on body weight, scrotal width, and sexual behavior of albino mice was evaluated for 30 days and all the outputs were described as shown below.
Effect on body weight following intratesticular injection of cetrimide
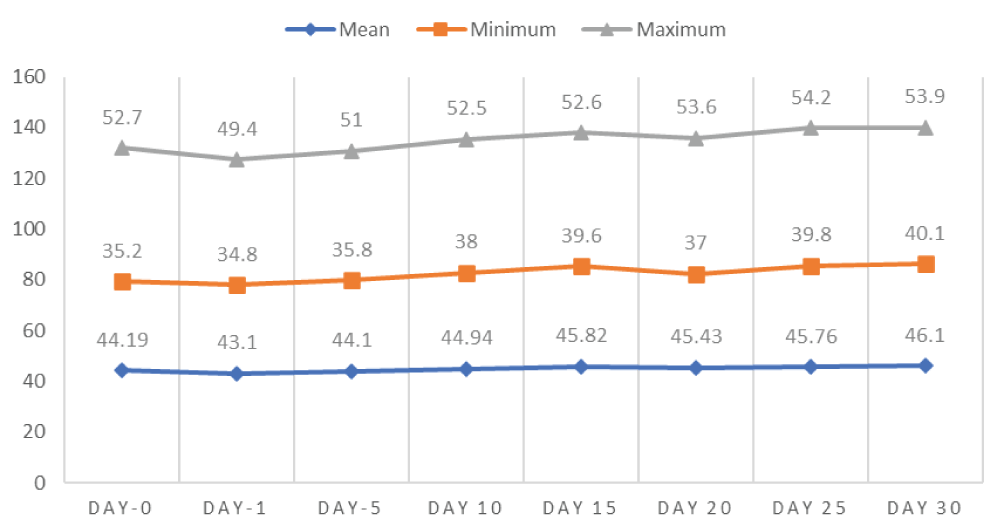
The effect of single bilateral intratesticular injection of cetrimide on body weight at doses of 5, 10, 15, and 20 mg/100 g bodyweight of each albino mice over the days of follow up is shown in figure 1. Accordingly, at the end of the experiment (day 30), there was an increase in the body weight (46.1 ± 3.94) of all albino mice in all cetrimide treated groups as well as in the control group. Thus, there was a significant increase (p - 0.0000) in the bodyweight of albino mice in all groups treated throughout the experiment after a single bilateral intratesticular injection of cetrimide 2% in comparison to the control group (Figure 1).
Figure 1: Effect of intratesticular injection of cetrimide on body weight (gram) over the experimental days.
Effect on scrotal width following intratesticular injection of cetrimide
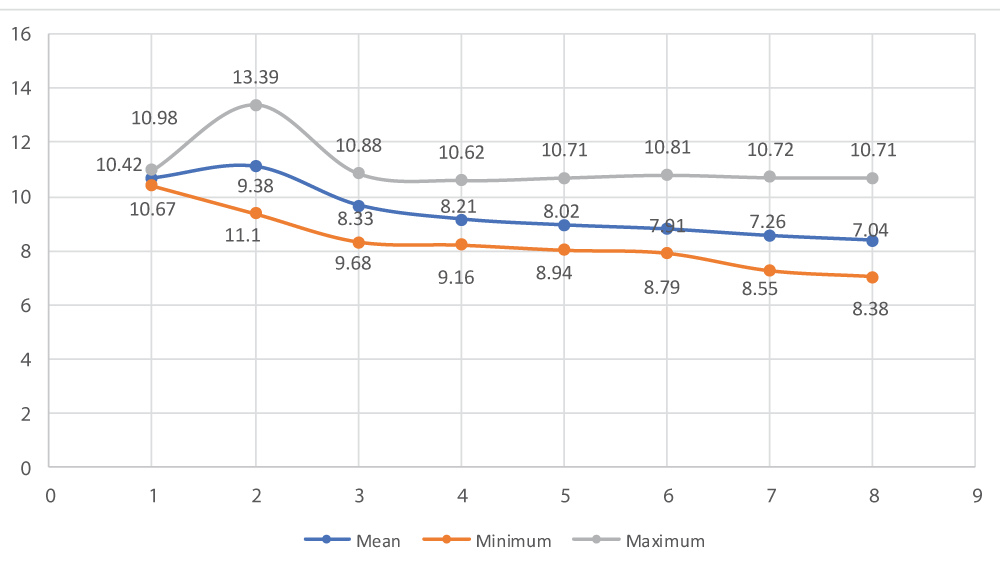
The effect of single intratesticular injection of cetrimide on scrotal width of albino mice at doses of 5, 10, 15, and 20 mg/100 g body weight over the days of follow up as compared to control groups is illustrated in figure 2. On day 30, there was a reduction in the mean scrotal width (8.38 ± 1.23) of all albino mice in all cetrimide treated groups. Hence, there was a significant (p - 0.0000) reduction in the mean scrotal width of albino mice at different doses of treatment as compared to the control group (Figure 2).
Figure 2: Effect of intratesticular injection of cetrimide on scrotal width (mm) over the experimental days.
Comparison of cetrimide used at different doses with respect to days of follow up
The mean comparison of cetrimide on both body weight and scrotal width of albino mice at different doses of treatment with respect to days of follow up was illustrated in table 1 and table 2. Accordingly, there was a significant (p - 0.04) increment of body weight in day 20 after intratesticular injection of cetrimide in all treatment groups (Table 1).
| Table 1: Comparison of mean bodyweight of experimental groups over days of treatment (Mean ± SD). | ||||||||
| Groups | Bodyweight over Experimental Days (gram) (Mean ± Std. Dev.) | |||||||
| Day-0 | Day-1 | Day-5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |
| Control (0.1 ml normal saline) |
44.43 ± 2.45 | 44.25 ± 2.32 | 44.53 ± 1.86 | 44.73 ± 1.86 | 44.85 ± 1.87 | 44.98 ± 1.91 | 44.55 ± 1.83 | 44.5 ± 1.98 |
| 5 mg/100 g BW/testis | 45.48 ± 2.28 | 44.8 ± 2.75 | 45.38 ± 2.59 | 46.23 ± 2.47 | 47.18 ± 1.95 | 47.6 ± 2.19** | 46.7 ± 3.68 | 46.8 ± 3.29 |
| 10 mg/100 g BW/testis | 47.68 ± 6.19 | 45.63 ± 4.93 | 47.43 ± 599 | 48.53 ± 5.76 | 49.28 ± 5.02 | 49.63 ± 5.62** | 49.63 ± 5.93 | 50.15 ± 5.64 |
| 15 mg/100 g BW/testis | 41.35 ± 4.72 | 40.25 ± 4.27 | 41.78 ± 4.29 | 42.78 ± 3.28 | 44.23 ± 3.44 | 41.63 ± 3.22** | 44.1 ± 3.11 | 44.73 ± 3.33 |
| 20 mg/100 g BW/testis | 42.03 ± 4.33 | 40.6 ± 3.60 | 41.28 ± 2.98 | 42.43 ± 3.49 | 43.55 ± 3.44 | 43.3 ± 3.32** | 43.8 ± 2.99 | 44.33 ± 2.84 |
| **p < 0.001. | ||||||||
As indicated in table 2, there was a significant reduction of scrotal width in albino mice in day 1 (p - 0.04), 10 (p - 0.0001), 15 (p - 0.0001), 20 (p - 0.0000), 25 (p - 0.0000) and 30 (p - 0.0000) with respect to each experimental groups. However, there was no significant reduction of scrotal width in albino mice before (day 0) intratesticular injection of cetrimide in all given doses of treatment (Table 2).
| Table 2: Comparison of Mean Scrotal Width of Experimental Groups over Days of Treatment (Mean ± SD). | ||||||||
| Groups | Scrotal width over Experimental Days (millimeter) (Mean ± Std. Dev.) | |||||||
| Day-0 | Day-1 | Day-5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 | |
| Control (0.1 ml normal saline) | 10.67 ± 0.23* | 10.85 ± 0.13** | 10.73 ± 0.13*** | 10.53 ± 0.08*** | 10.63 ± 0.07**** | 10.72 ± 0.08**** | 10.69 ± 0.04**** | 10.65 ± 0.05**** |
| 5 mg/100 g BW/testis | 10.21 ± 0.54* | 10.85 ± 0.54** | 9.63 ± 0.22*** | 8.86 ± 0.68*** | 8.34 ± 0.31**** | 8.12 ± 0.18**** | 7.75 ± 0.33**** | 7.68 ± 0.10**** |
| 10 mg/100 g BW/testis | 9.55 ± 1.6* | 12.30 ± 1.32** | 8.57 ± 0.24*** | 8.47 ± 0.25*** | 8.29 ± 0.25**** | 8.19 ± 0.20**** | 7.89 ± 0.29**** | 7.49 ± 0.43**** |
| 15 mg/100 g BW/testis | 9.91 ± 0.87* | 10.57 ± 0.81** | 9.54 ± 0.73*** | 8.78 ± 0.57*** | 8.54 ± 0.58**** | 8.29 ± 0.59**** | 8.02 ± 0.44**** | 7.84 ± 0.49**** |
| 20 mg/100 g BW/testis | 9.48 ± 0.66* | 10.92 ± 0.38** | 9.98 ± 0.44*** | 9.15 ± 0.29*** | 8.89 ± 0.35**** | 8.63 ± 0.23**** | 8.38 ± 0.29**** | 8.25 ± 0.23**** |
| * p > 0.05, **p < 0.05, *** p < 0.001; **** p < 0.00001. | ||||||||
Effect on histopathology of testicular tissue
Histopathological examination of testicular tissue was performed at the end of the experiment (day 30) after the humane sacrifice of each albino mice. Thus, testicular samples were taken from each experimental albino mice from each group of treatment to assess the effect of cetrimide 2% following single bilateral intratesticular injection. Routine histopathological processing and staining of samples were performed. The qualitative assessment of testicular sections and histological architecture of seminiferous tubules, interstitial cells, and germ cell arrangement in tubules of each sample were performed and seen under the light microscope. Accordingly, different histopathological findings of testicular tissue were recorded.
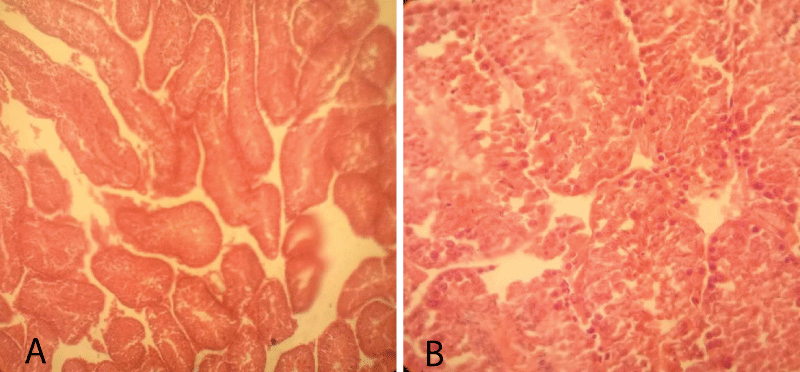
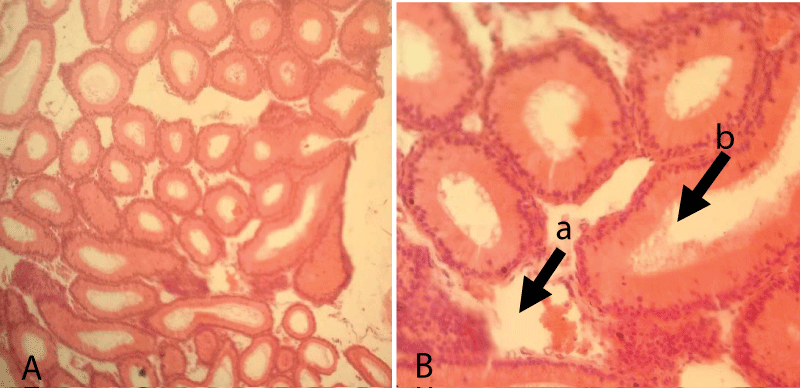
The histopathological finding of group-1 revealed that the seminiferous tubules including the germ cells were intact and cellular structures were clearly identified. Besides, different levels of spermatid, Sertoli cells, Leydig, and interstitial cells were also intact. Thus, testicular sections showed normal arrangements of germ cells in seminiferous tubules with distinct peritubular space and interstitial cells in the control group (Figure 3).
Figure 3: Testicular cross-section of the control group showing the normal arrangement of germ cells in seminiferous tubules [10x (A) and 40x (B), respectively].
Histopathological finding group-2 indicated that degeneration in some intratubular cells and the appearance of few necrotized areas in some parts of seminiferous tubules. The sloughing of immature germ cells from the basement membrane into the intraluminal space was noted. Exfoliation of germ cells in the tubules was observed. However, there were no thickening or inflammation of interstitial cells and most of the germ cells and seminiferous tubules were normal; yet, in some of the seminiferous tubules were necrotized and become empty (Figure 4).
Figure 4: Testicular cross-section of 5 mg cetrimide-treated mice showing few degenerative and necrosis of germ cells in seminiferous tubules (Arrows indicate the area of necrosis in seminiferous tubule along with immature germ cell detachment from the basement membrane.) [10x (A) and 40x (B), respectively].
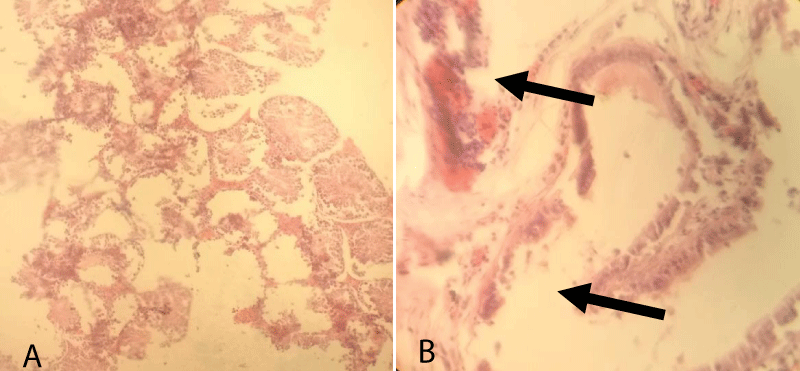
According to histopathological finding group-3, there was a remarkable degree of coagulative necrosis along with fibrosis in the seminiferous tubules. The blood vessels in the interstitial space were hyperemic (congested), dilated, and were full of RBCs. Besides, there was also little interstitial thickening and sloughed into the lumen. In seminiferous tubules, the intratubular cells were slightly degenerated, some necrotized and sloughed into the lumen and some tubules were almost left empty and severely necrotized. However, some seminiferous tubules, interstitial cells including the Leydig cells showed normal arrangement (Figure 5).
Figure 5: Testicular cross-section of 10 mg cetrimide-treated albino mice showing coagulative necrosis (arrow, b) and hyperemic due to the blood vessels (arrow, a) in the interstitial space and fibrosed seminiferous tubules. [10x (A) and 40x (B), respectively].
In group 4, the histopathological finding of testicular tissue revealed that there was severe necrosis of testicular parenchyma and atrophied seminiferous tubules. Moreover, most of the tubules were totally distorted since there was no concrete demarcation between the tubular and extra tubular zone. In all tubules, the intratubular cells including the Sertoli cells were necrotized and the tubules were empty. Prominent degeneration and necrosis of interstitial cells were and fibrosis of the Leydig cells was evident (Figure 6).
Figure 6: Testicular cross-section of 15 mg cetrimide-treated albino mice showing a high degree of degenerated germ (arrow, b) cells into the tubules. Prominent degeneration and necrosis of interstitial cells were and fibrosis of the Leydig cells (arrow, a) was evident [10x (A) and 40x (B), respectively].
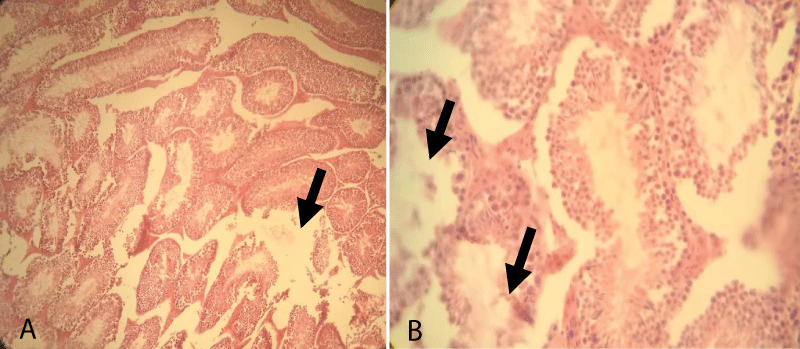
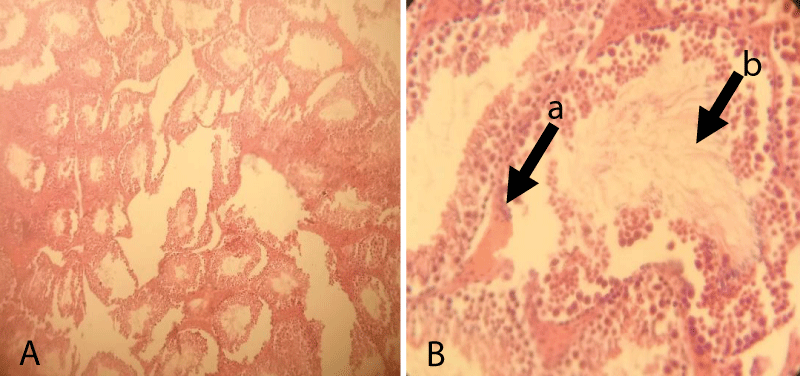
The histopathological finding of Group-5 showed that all tissues were necrotized and even it was difficult to identify the structure within testicular tissue. Besides, complete derangement of tubular architecture without any distinct boundary between the tubular and extra tubular compartment along with the infiltration of a large number of leucocytes throughout the testicular tissue was noted. The notable appearance of the fibrous tissue of germ cells was also noted throughout the testicular sections (Figure 7).
Figure 7: Testicular cross-section of 20 mg cetrimide-treated albino mice showing complete derangement of seminiferous tubules (Arrow) and infiltration of leucocytes and the presence of fibrous tissue throughout the tubular and extra tubular zone [10x (A) and 40x (B), respectively].
Evaluation of sexual behavior and fertility performance
There were drastic changes noted in the sexual behavior of the cetrimide-treated albino mice in comparison to the control of albino mice. All albino mice were treated with different doses of cetrimide solution and those who were treated with a high dose that is 15 and 20 mg Cetrimide did not show sexual desire to mate with the female one. The fertility performance of males were evaluated by seeing the pregnancy status of female albino mice after mating and kept in a cage separately for 21 days.
On the 21st post-coital day, four female albino mice were kept with male albino mice which were treated with different doses that are 5, 10, 15, or 20 mg. Accordingly, all the male albino mice that were kept in group five (20 mg/100 g body weight) were unable to mate with those co-caged female albino mice and no pregnancy was recorded in this group. However, all females that were allowed to mate with control males showed a 100% pregnancy rate 21 days post-coital (Table 3).
| Table 3: Fertility performance in mature male albino mice four weeks after single bilateral intratesticular cetrimide injection. | ||
| Sexual Behavior | No. of males mated/co-caged | No. of females mated/ pregnant |
| Female mated with control male (4)* | 4/4 | 4/4 |
| Female mated with 5 mg/100 g BW Cetrimide treated male (4)* | 3/4 | 3/1 |
| Female mated with 10 mg/100 g BW Cetrimide treated male (4)* | 2/4 | 2/0 |
| Female mated with 15 mg/100 g BW Cetrimide treated male (4)* | 1/4 | 1/0 |
| Female mated with 20 mg/100 g BW Cetrimide treated male (4)* | 0/4 | 0/0 |
| *indicates the number of males used for mating purposes in each treatment group. | ||
Intratesticular injections of chemicals have been investigated as a method of contraception in different animals for more than five decades. However, this experimental study is the first to show a potent effect of cetrimide following intratesticular injection in male albino mice and its histological changes. Although surgical castration is the most effective method, injection of various chemical agents into the epididymis can help to limit male reproduction [3,18,45] and testes [46]. In the present study, a single intratesticular injection of cetrimide at high doses (15 and 20 mg) caused severe necrosis and degeneration of testicular tissue in albino mice.
Several systemic routes of administration such as intraperitoneal, subcutaneous, intravenous, and oral have been used for delivering agents to the testes to study their effects on spermatogenesis. However, intratesticular injection is a technique that might offer some potential benefits, over other routes of administration, in the study of fertility control or regulation of spermatogenesis [2,22,23,25,32,47]. This method may be used to study the direct effect of the chemicals on the testis with unknown toxicity and such agents are not systemically metabolized. A variety of chemical sterilants have been developed for injection into the testis of albino mice, which were either safe but not effective or vice versa [16,17,48].
Numerous investigators have employed intratesticular injections to study the chemosterilization effect of a variety of agents [3,23,25,47]. Russell, et al. in 1987 studied the technique of intratesticular injection and found that a sterile 26-gauge needle can be used and has no effects on the histology of the seminiferous tubules. The study also suggested saline, ethylene glycol, dimethyl sulfoxide (DMSO) mixed 1:1 with saline and propylene glycol to be suitable injection chemicals because they did not affect testicular histology [3,49].
In this experimental study, a dose-dependent cetrimide induced chemical sterilization was conducted to assess the effect of single bilateral intratesticular injection of cetrimide 2% on body weight, scrotal width, and sexual behavior of albino mice. Besides, histopathological examinations of testicular tissue were performed to investigated and assess the histological change on the testicular tissue. All experimental groups of mice were clinically monitored for 30 days. All treated albino mice tolerated the intratesticular injections of cetrimide and there was no apparent discomfort, distress, and inflammatory swelling of the testis. Moreover, no signs of morbidity or mortality were exhibited in all experimental groups of mice during the time of the experiment. This finding is in agreement with previous reports by different authors [2,3,14,16,17,25,32].
Accordingly, the effect of intratesticular injection of cetrimide 2% on body weight and scrotal width at doses of 5, 10, 15, and 20 mg in albino mice were conducted. There was a significant increase in body weight in day 20 and also a significant reduction in the mean scrotal width in days 1, 10, 15, 20, 25, and 30 of albino mice in all experimental groups were observed. Besides, reduction in size during scrotal palpation after intratesticular injection cetrimide 2% is due to severe degeneration of the testicular tissue, that might be rendered the experimental mice infertile. The initial testicular swelling noted in this study might be due to inflammatory edema. The testicular atrophy could be due to necrosis of the testicular tissue and testicular gland parenchyma. These observations are in agreement with previous studies with Calcium chloride on the testis in the rat [16,17]. Correspondingly, similar findings were recorded in other domestic animals by [5,12,25,50].
The histopathology result revealed, degenerative changes associated with graded doses of cetrimide. Disintegration, degeneration, and necrosis of germ cells in seminiferous tubules were noted even with the lowest dose (5 mg), although the tubular compartment remained intact. Drastic necrosis in seminiferous tubules along with atrophy of the tubules was noted at 10mg dose of cetrimide though the tubular compartment was intact. But, after high doses (15 or 20 mg) of cetrimide treatment, complete degeneration of germ cells with the absence of a distinct boundary of seminiferous tubules and fibrosis of the interstitial spaces was observed. These changes might be due to the necrotizing property of cetrimide that cause extensive degeneration on the germ cells, seminiferous tubules, and interstitial cells. This finding is comparable with results reported by others [3,5,17,23,25,51,52] who have used the same experimental protocol; but, a different chemical was used.
Furthermore, this study demonstrated that intratesticular injection of the cetrimide altered the structure and function of the male reproductive organs including degeneration and necrosis of seminiferous germ cells, morphological changes in Sertoli and Leydig cells and atrophy of interstitial and germ cells. Based on these changes, it can be concluded that intratesticular injection of cetrimide caused infertility. After cetrimide treatment, complete degeneration of germ cells, absence of a distinct boundary in the seminiferous tubules and fibrosis, and appearance of hyaline tissue were observed in the interstitial spaces. These changes may be due to the necrotizing properties of cetrimide. Such similar changes were reported by other researchers using calcium chloride, hypertonic saline solution, and salvon [2,3,12,19,23,25,32,48,53].
Degenerative changes and total disintegration of germ cells at doses of 15 and 20 mg were also observed in the seminiferous tubules, interstitial and Leydig cells. The degeneration of germ cells in the cetrimide-treated testis is possibly mediated by apoptosis. This is supported by germ cell detachment from the basal lamina, exfoliated germ cells, and infiltration of leucocytes in the seminiferous tubules, necrosis of interstitial and Leydig cells and sloughing of immature germ cells in the intraluminal space. This result is inconsistent with other findings [12,17,19,22,23,32,51,53-58].
At high dose (15 and 20 mg) of cetrimide treatment, peritubular space in testicular sections of cetrimide-treated mice also exhibited significant degenerative changes along with the proliferation of fibrous tissue. Moreover, fibrosis of peritubular space in experimental mice is also consistent with previous studies using other chemical agents [14,17,21,23,32,34]. The infiltration of leucocytes into the tubular and peritubular zones is possibly due to the degeneration of germ cells, which may release a large number of proteins that defuse out to the luminal cavity of the seminiferous tubule and into the interstitium where they act as antigen to promote chemotaxis of leucocytes [2,3,17,25,32,35].
To confirm the induction of successful chemosterilization by single intratesticular injection of cetrimide, the fertility efficacy test was performed. A significant diminution in scrotal width and gross reduction on testicular size was noted in all experimental groups except the control group. In contrast, there was no pregnancy in female albino mice after mating with male mice treated at doses of 10, 15, or 20 mg. This might be related to the necrosis and degenerative change of germ cells that can result in a lack of a minimum required number of motile and fertile sperm in treated males [3,16,22,26,35,59].
In conclusion, the only proven methods so far for the sterilization of male animals are orchiectomy and vasectomy. However, both methods require anesthesia, adequate surgical facilities with skilled veterinarian and equipment, and postoperative care. Thus, a single bilateral intra-testicular injection of cetrimide is effective, economical, and easy to perform and does not require the removal of testis. To sum up, this agent may be used as a sterilizing agent to manage the uncontrolled growth of mammals like stray dogs, monkeys after further investigation.
An experimental study was conducted to evaluate the effect of single bilateral intratesticular injection of cetrimide 2% on body weight, scrotal width, and sexual behavior of albino mice. Moreover, histopathological evaluations of testicular tissue were also performed. The current study revealed a significant increase in body weight in all the experimental albino mice after day 20 and there was also a significant reduction of scrotal width in albino mice after intratesticular injection of cetrimide in day 1, 10, 15, 20, 25 and 30 in each experimental groups. On the basis of testicular histology, disintegration, degeneration, and necrosis of germ cell in seminiferous tubules were noted even at the lowest dose. However, after injection cetrimide 2% at doses of 15 or 20 mg, complete degeneration of germ cells, absence of a distinct boundary of seminiferous tubules, the fibrosis of the interstitial were observed. Besides, drastic alterations in the sexual behavior of the treated males were observed in the present study with the loss of libido especially at a dose of 20 mg cetrimide-treated animals.
Thus, further study should be performed with a larger number of experimental animals and longer duration to verify total security and absence of fertility recovery. Moreover, the mechanism of infertility is not clear on the current study; so further assessment should be done in the future. Further investigation on the determination of the weight of sex organs, blood testosterone and blood cortisol level, semen volume, and testicular androgenic enzymes should be conducted.
I owe my gratitude to my sincere advisor Dr. Guesh Negash for his constructive comments and guidance during my thesis write up and I would like to thank Dr. Tilaye Demisse for his assistance during my histopathology result interpretation and Mr. Kidane Werkeleul for his support during my pathology laboratory work. My special thanks goes to my best friends and my families for their incalculable moral support during my research work.
- Auer JA, Stick J. Reproductive system in equine surgery. 3rd ed. Equine surgery, ed. In: Auer JA, editors. . 2006, USA: Saunders Elsevier. 282-295.
- Ibrahim A. Evaluation of chemical castration with calcium chloride versus surgical castration in donkeys: testosterone as an endpoint marker. BMC Vet Res. 2016; 12: 46.
- Jana K, Samanta PK. Sterilization of male stray dogs with a single intratesticular injection of calcium chloride: a dose-dependent study. Contraception. 2007; 75: 390-400. PubMed: https://pubmed.ncbi.nlm.nih.gov/17434022/
- Fesseha H. Non-surgical sterilization methods in male animals: A review. Vet Med Open J. 2019; 4: 49-56.
- Leoci R, Aiudi G, Silvestre F, Lissner EA, Marino F, et al. A dose-finding, long-term study on the use of calcium chloride in saline solution as a method of nonsurgical sterilization in dogs: evaluation of the most effective concentration with the lowest risk. Acta Vet Scand. 2014; 56: 63. PubMed: https://pubmed.ncbi.nlm.nih.gov/25317740
- Matsumoto AM. Is high dosage testosterone an effective male contraceptive agent? Fertility and sterility, 1988. 50: 324-328. PubMed: https://pubmed.ncbi.nlm.nih.gov/3396702/
- Swerdloff R, Wang C, Bhasin S. 10 Developments in the control of testicular function. Bailliere's clinical endocrinology and metabolism. 1992; 6: 451-483. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4801682/
- Dube D, Assaf A, Pelletier G, Labrie F. Morphological study of the effects of an GnRH agonist on the canine testis after four months of treatment and recovery. Eur J Endocrinol, 1987; 116: 413-417.
- Wu FC, Aitken RJ. Suppression of sperm function by depot medroxyprogesterone acetate and testosterone enanthate in steroid male contraception. Fertil Sterility. 1989; 51: 691-698.
- Tremblay Y, Bélanger A. Reversible inhibition of gonadal functions by a potent gonadotropin-releasing hormone agonist in adult dog. Contraception. 1984; 30: 483-497.
- Gonzalez A. Immunological approaches to contraception in dogs. J Reproduct Ferti Suppl. 1989; 39: 189-198.
- Leoci R, Aiudi G, Silvestre F, Lissner EA, Lacalandra GM. Alcohol diluent provides the optimal formulation for calcium chloride non-surgical sterilization in dogs. Acta Vet Scand. 2014; 56: 62.
- Kar AB, Kamboj V, Goswami A. Sterilization of male rhesus monkeys by iron salts. Reproduction. 1965; 9: 115-117.
- Gebremeskel H. Chemosterilization Effct of Single Intratesticular Injection of Chlorhexidine in Male Adult Albino Mice-A Histopathological Study. J Tissue Biol Cytol. 2020; 3: 005.
- Emir L. Hormonal and pathologic changes after chemoablation of testes with hypertonic saline solution as a treatment method alternative to orchiectomy in patients with hormone sensitive metastatic prostatic cancer. Urologic Oncology: Seminars and Original Investigations. 2011; 29: 212-217.
- Jana K, Samanta PK, Ghosh D. Dose-dependent response to an intratesticular injection of calcium chloride for induction of chemosterilization in adult albino rats. Vet Res Community. 2002; 26: 651–673. PubMed:
- Jana K, Samanta PK. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception. 2006; 73: 289-300. PubMed: https://pubmed.ncbi.nlm.nih.gov/16472573/
- Nishimura N, Kawate N, Sawada T. Chemical castration by a single intratesticular injection of lactic acid in rats and dogs. Journal of Reproduction and Development. 1992; 38: 263-266. PubMed:
- Oliveira EC, Moura MRP, de Sá MJC, Silva Jr VA, Kastelic JP, et al. Permanent contraception of dogs induced with intratesticular injection of a zinc gluconate-based solution. Theriogenology. 2012; 77: 1056-1063. PubMed: https://pubmed.ncbi.nlm.nih.gov/22192397/
- Pineda M, Reimers TJ, Faulkner LC, Hopwood ML, Seidel Jr, GE. Azoospermia in dogs induced by injection of sclerosing agents into the caudae of the epididymides. Am J Vet Res; 1977; 38: 831-838. PubMed: https://pubmed.ncbi.nlm.nih.gov/560154/
- Immegart HM, Threlfall WR. Evaluation of intratesticular injection of glycerol for nonsurgical sterilization of dogs. Am J Vet Res. 2000; 61: 544-549. PubMed: https://pubmed.ncbi.nlm.nih.gov/10803650/
- Abshenas, J, Molaei MM, Derakhshnfar A, Ghalekhani N. Chemical Sterilization by Intratesticular Injection of Eugenia Caryophyllata Essential Oil in Dog: A Histopathological Study. Iranian J Vet Surg. 2013; 8: 9-16.
- Canpolat I, Karabulut E, Eröksüz Y. Chemical castration of adult and non-adult male dogs with sodium chloride solution. Journal of Agri Vet Sci. 2016; 9: 9-11.
- Pineda M, Dooley M. Surgical and chemical vasectomy in the cat. Am J Vet Res. 1984; 45: 291-300. PubMed: https://pubmed.ncbi.nlm.nih.gov/6711952/
- Jana K, Samanta PK. Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride. BMC Vet Res. 2011; 7: 39. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3152893/
- Fagundes AKF, Oliveira ECS, Tenorio BM, Melo CCS, Nery LTB. et al. Injection of chemical castration agent, zinc gluconate, into the testes of cats results in the impairment of spermatogenesis: A potentially irreversible contraceptive approach for this species. Theriogenology. 2014; 81: 230-236. PubMed: https://pubmed.ncbi.nlm.nih.gov/24238399/
- Neto OA, Gasperin BG, Rovani MT, Ilha GF, Nóbrega Jr JE, et al. Intratesticular hypertonic sodium chloride solution treatment as a method of chemical castration in cattle. Theriogenology. 2014; 82: 1007-1011. PubMed: https://pubmed.ncbi.nlm.nih.gov/25149022/
- Canpolat I, Gur S, Gunay C, Bulut S. An evaluation of the outcome of bull castration by intra-testicular injection of ethanol and calcium chloride. Revue de Méd Vét. 2006; 157: 420.
- Capucille DJ, Poore MH, Rogers GM. Castration in cattle: techniques and animal welfare issues. Compendium, 2002. 24: 66-73.
- Ijaz A, Abalkhail A, Khamas W. Effect of intra testicular injection of formalin on seminiferous tubules in Awassi lambs. Pakistan Vet J. 2000; 20: 129-134.
- Jana K, Samanta PK, Ghosh D. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization of male Black Bengal goats (Capra hircus): a dose-dependent study. Anim Reprod Sci. 2005; 86: 89-108. PubMed: https://pubmed.ncbi.nlm.nih.gov/15721661/
- Mohammed A, James F. Chemical castration by a single bilateral intra-testicular injection of chlorhexidine gluconate and cetrimide in bucks. Sokoto J Vet Sci. 2013; 11: 62-65.
- >Hassan A, Fromsa A. Review on Chemical Sterilization of Male Dogs. Int J Adv Res. 2017; 5: 758-770.
- Pařizek J. The third oliver bird lecture sterilization of the male by cadmium salts. Reproduction. 1960; 1: 294-309.
- Heath E, Arowolo R. The Early Histopathologic Effects of Intratesticular Injection with Hyperosmolar Glycerol, Glucose or NaCI Solutions. Rologia. 1987; 19: 654-661. PubMed: https://pubmed.ncbi.nlm.nih.gov/3434855/
- Fordyce G, Hodge PB, Beaman NJ, Laing AR, Campero C, et al. An evaluation of calf castration by intra-testicular injection of a lactic acid solution [beef cattle]. Aust Vet J.1989; 66 272-276. PubMed: https://pubmed.ncbi.nlm.nih.gov/2684125/
- Alper B, İbrahim C. Chemical sterilization in domestic animals. Research in Agricultural and Veterinary Sciences. 2019; 3: 5-9.
- Wiebe JP, Barr KJ, Buckingham KD. Sustained azoospermia in squirrel monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception. 1989; 39: 447-457.
- NIH, Guide for the care and use of laboratory animals, in National Institute of Health. Bethesda (Md) 7 US Department of Health and Human Services. 2010.
- NRC, Guidelines for care and use of animals in scientific research, in National Research Council. National Academies Press: New Delhi (India) Indian National Science Academy. 2002.
- Petrie A, Watson. Statistics for veterinary and animal science. Balckwell Science Ltd.: Malden, USA.1999.
- Woodall P, Johnstone I. Scrotal width as an index of testicular size in dogs and its relationship to body size. Journal of Small Animal Practice, 2008. 29: 543-547.
- Talukder M. Histopathology Techniques: Tissue Processing and Stainning. 2007: http://www.talukderb.com .
- Fox KA, Diamond B, Sun F, Clavijo A, Sneed L, et al. Testicular lesions and antler abnormalities in Colorado, USA mule deer (Odocoileus hemionus): a possible role for epizootic hemorrhagic disease virus. J Wildlife Dis. 2015; 51: 166-176. PubMed: https://pubmed.ncbi.nlm.nih.gov/25375947
- Freeman C, Coffey D. Sterility in male animals induced by injection of chemical agents into the vas deferens. Ferti Sterility. 1973; 24: 884-890. PubMed: https://pubmed.ncbi.nlm.nih.gov/4742009/
- Hill G, Neville JW, Richardson K. Castration Method and Progesterone-Estradiol Implant Effects on Growth Rate of Suckling Calves. J Dairy Sci. 1985; 68: 3059-3061.
- Igdoura S, Wiebe J. Suppression of Spermatogenesis by Low Level of Glycerol Treatment. J Androl. 1994; 15: 234-243.
- Oliveira E, Moura M, Silva JV. Intratesticular injection of a zinc-based solution as a contraceptive for dogs. Theriogenology. 2007; 68: 137-145.
- Russell L, Saxena N, Weber J. Intratesticular injection as a method to assess the potential toxicity of various agents and to study mechanisms of normal spermatogenesis. Gamete Res. 1987; 17: 43-56. PubMed: https://pubmed.ncbi.nlm.nih.gov/2906900/
- Karmakar SN, Das SK. Chemosterilization induced by intratesticular injection of calcium chloride (CaCl2)-a tool for population control. Int J Pharmaceut Che Biol Sci. 2017; 7: 25-35.
- Cavalieri J. Chemical sterilisation of animals: A review of the use of zinc-and CaCl2 based solutions in male and female animals and factors likely to improve responses to treatment. Anim Reprod Sci. 2017; 181: 1-8. PubMed: https://pubmed.ncbi.nlm.nih.gov/28366279/
- McGinnis K, Wangi K, Genegy M. Alterations of extra-cellular calcium elicits selective modes of cell death and protease activation in SH-SY5Y human neuroblastoma cells. J Neurochem. 1999; 72: 1853– 1863. PubMed: https://pubmed.ncbi.nlm.nih.gov/10217261
- Oliveira EC, Fagundes AKF, Meloc CCS, Nery LTB, Rêvoredo RG, et al. Intratesticular injection of a zinc-based solution for contraception of domestic cats: a randomized clinical trial of efficacy and safety. Vet J. 2013; 197: 307-310.
- Doerksen T, Bonoit G, Trasler J. Deoxyribonucleic acid hypomethylation of male germ cells by mitotic and meiotic exposure to 5-azacytidine is associated with altered testicular histology. Endocrinology. 2000; 141: 3235-3244. PubMed: https://pubmed.ncbi.nlm.nih.gov/10965894/
- Emir L, Dadali M, Sunay M, Erol D, Caydere M, et al. Chemical castration with intratesticular injection of 20% hypertonic saline: A minimally invasive method. Urol Oncol. 2008; 26: 392-396. PubMed: https://pubmed.ncbi.nlm.nih.gov/18367099/
- Tillbrook A, Clarke I. Negative feedback regulation of the secretion and actions of gonadotrophin releasing hormone in males. Biol Reprod. 2001. 64: 735– 742. PubMed: https://pubmed.ncbi.nlm.nih.gov/11207186/
- Xu C, Lu MG, Feng JS, Guo QS, Wang YF. Germ cell apoptosis induced by Ureaplasma urealytium infection. Asian J Androl. 2001; 3: 199-204. PubMed: https://pubmed.ncbi.nlm.nih.gov/11561190/
- Sharpe R, Maddocks S, Millar M, Kerr JB, Saunders PT, et al. Testosterone and Spermatogenesis Identification of Stage‐Specific, Androgen‐Regulated Proteins Secreted by Adult Rat Seminiferous Tubules. J Androl. 1992; 13: 172-184. PubMed: https://pubmed.ncbi.nlm.nih.gov/1317835/
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2, 4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001; 64: 1500-1508. PubMed: https://pubmed.ncbi.nlm.nih.gov/11319158/