More Information
Submitted: March 09, 2023 | Approved: April 11, 2023 | Published: April 12, 2023
How to cite this article: Florez CO, Torres LEC, de Oliveira JG, Lobato HC, da Fonseca LA, et al. Comparative characterization between autologous serum and platelet lysate under different temperatures and storage times. Insights Vet Sci. 2023; 7: 001-009.
DOI: 10.29328/journal.ivs.1001038
Copyright License: © 2023 Florez CO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Comparative characterization between autologous serum and platelet lysate under different temperatures and storage times
Camilo Osorio Florez*  , Luís Ernesto Campos Torres, Jéssica Guerra de Oliveira, Henrique Carneiro Lobato, Leandro Abreu da Fonseca, Andrés Ortega Orozco, Fabíola de Oliveira Paes Leme, Priscila Fantini and Renata de Pino Albuquerque Maranhão
, Luís Ernesto Campos Torres, Jéssica Guerra de Oliveira, Henrique Carneiro Lobato, Leandro Abreu da Fonseca, Andrés Ortega Orozco, Fabíola de Oliveira Paes Leme, Priscila Fantini and Renata de Pino Albuquerque Maranhão
Animal Science, Federal University of Minas Gerais, Belo Horizonte, Brazil
*Address for Correspondence: Camilo Osorio Florez, Animal Science, Federal University of Minas Gerais, Belo Horizonte, Brazil, Email: [email protected]
Therapies using autologous serum and platelet lysate have shown promise among blood and biological products in the treatment of various diseases. The autologous serum has been shown to be a superior alternative to traditional eye drops in treating eye diseases in ophthalmology. Figurelet lysate (PL) has recently been considered a more interesting alternative for the treatment of multiple tissues, as it does not have the unfavorable reactions seen with traditional platelet-rich plasma (PRP), making it a valuable blood derivative for use in ocular therapy. There is no definitive comparison in veterinary medicine between PL and autologous serum in terms of the content of Transforming Growth Factor beta 1 (TGF-1), which is known to have chemotactic, mitogenic, matrix formation, and angiogenesis effects on tissues, and beneficial proteins in ocular tissue. This study aimed to estimate the concentrations of TGF-1, total protein, and albumin, as well as autologous serum and platelet lysate, in horses over an 8-day storage period at temperatures of 4 °C and 37 °C. To produce autologous serum, 63 ml of blood was collected from each animal in seven 9 ml tubes without anticoagulant. For platelet lysate, 180 ml of blood was collected in 50 tubes of 3.6 ml with 3.2% sodium citrate. The most significant findings were the positive relationship between the baseline platelet count in the blood and the final platelet concentration in PRP. Specifically, we found a correlation (R = 0.9) with a p - value of 0.005 between the average baseline platelet level of seven animals and their corresponding PRP results, both on an individual level and as a group. Additionally, there was a correlation between growth factor concentration and PRP platelets, with the highest growth factor concentration in PL. The temperature storage group exhibited higher concentrations of total protein and serum albumin, as well as the maximum amount of growth factor for both products at a temperature of 37 °C.
Throughout history, veterinary medicine has sought therapeutic alternatives for the correct management of different lesions in animals. Blood products and platelet concentrates have gained popularity due to their significant effects on the speed and quality of tissue repair [1].
Figurelet-rich plasma (PRP) and autologous serum (AS) are two of the most used blood products. PRP is a product obtained by centrifugation of whole blood with an anticoagulant, rich in platelets, cytokines, and growth factors [2]. Likewise, AS is a blood product resulting from sedimentation and/or centrifugation of whole blood without anticoagulant [3]; rich in growth factors and other molecules with beneficial properties for tissue repair [4,5]. Growth factors are molecules that promote the wound-healing process and stimulate the regenerative capacity of affected tissues, due to the increase in mitosis and consequent hyperplasia of the epithelium [6,7].
AS contains growth factors and other molecules that have beneficial properties for tissue repair, with an emphasis on ophthalmic diseases [8]. PL is a biological product with no cellular composition, that has received little attention in veterinary medicine but with evident potential in human medicine. Like PRP, PL has the potential to aid in tissue repair, but with a lower immunogenic capacity [9,10]. Studies comparing the various compounds of SA and PL have not yet been conducted, nor is it known whether storage, aimed at use during ophthalmic treatment, can affect the Studies comparing various AS and PL compounds have not yet been conducted, nor is it known whether storage, aimed at use during ophthalmic treatment, can affect the product’s quality.
The detection of GFs has been performed mostly using commercial ELISA kits, previously validated for horses in previous studies [11-13]. Among the GFs studied, TGF-β1 is known to have chemotactic, mitogenic and extracellular matrix synthesis effects in tissues [14].
However, one of the factors that negatively affect the use of these blood products is the lack of knowledge about the concentrations of some growth factors, as well as the content of beneficial proteins in the tissues. Thus, the objective of this study was to demonstrate and compare the effects of storage under different temperatures in the aspects of protein composition and stability and the growth factor TGF-β1 of AS and PL, aiming at the use of a safe and characterized product concerning the beneficial compounds for the eye and different tissues in the equine species.
The experiment was carried out with the approval of the animal ethics committee of the Federal University of Minas Gerais (UFMG), Brazil under the internal protocol number CEUA: 232/2020. This statement indicates that the experiment was conducted following the guidelines and regulations set forth by the animal belonging to the phylum Chordata, subphylum Vertebrata (except man) for the scientific research purposes ethics committee of the university.
The experiment used the structure available in the MULTI LAB laboratory of the DCCV, LAMICO of the Preventive Veterinary Medicine Department, and LEPET in the Veterinary Hospital of the UFMG Veterinary School
Animals
Seven adult jumping horses (males and females), with a median age of 7-15 years, from the metropolitan region of Belo Horizonte, Minas Gerais, Brazil, were used. The animals were housed in equestrian centers nearby (15-30 minutes) to the place where the blood products were processed. The stables are approximately 3 x 3 meters in size, water ad libitum, mineral salt, feed based on grass hay, and concentrate. The animals were not removed from their environment or had their routine changed by the study.
Exclusion criteria in the study were: recent use of anti-inflammatory drugs and antibiotics, recent systemic illness (within the last month), recent travel (within the last month), or any change in the initial complete clinical examination.
Blood collection
Blood was collected from each animal, through the vacuum tube system, in the external jugular vein with a scalp for collecting 23 G-7” butterfly blood (vacuplast collect line, São Paulo, Brazil), following antisepsis by chlorhexidine degerming and alcoholic chlorhexidine.
To perform the hemogram, 4 mL vacuum tubes with 15% EDTA K3 (Biocon, Nova Lima, Minas Gerais, Brazil) were used. To prepare the PRP 3.6 mL tube with 3.2% sodium citrate (Biocon, Nova Lima, Minas Gerais, Brazil), A 9 mL vacuum dry tube without anticoagulant (Biocon Nova Lima, Minas Gerais, Brazil) was used to prepare the serum.
Preparation of autologous serum
To produce AS, 63 mL of blood was collected per animal in 7 tubes of 9 ml without anticoagulant. After collection, each tube was left in a vertical position for 1-2 hours at room temperature to facilitate natural clot retraction, followed by centrifugation (Centribio, 80-2B, Biovera) at 2500 g for 10 minutes to obtain an even more refined product, based on the Sanak protocol [15].
Preparation of platelet-rich plasma
For the PL, 50 tubes with sodium citrate 3.2% volume of 3.6 ml were collected for a total of 180 ml per animal, the tubes’ contents were transferred to 15 ml Falcon tubes, which were subjected to centrifugation for 5 minutes at 120 g (Centribio, 80-2B, Biovera). The resulting product was left to stand for 10 minutes; of this product, 50% of the plasma closest to the buffy coat was collected with a 1 ml pipette. This product was transferred in sterile 15 mL Falcon tubes and centrifuged again at 240 g for 5 minutes. Then, it was left to rest for another 10 minutes, before discarding 75% of the supernatant. The remaining 25% volume was called PRP. It was necessary to undo and homogenize the platelet pellet. This protocol was based on adaptations of the technique described by Arguelles [12]. The product was subjected to a lysis process.
Figurelet lysate
Following the removal of 25% of each tube, the product was transferred into a 15 mL falcon tube and subjected to a platelet lysate process. Tubes with PRP were subjected to three cycles of freezing at -80 °C for 30 minutes, followed by three cycles of thawing in a water bath at 37 °C for 15 minutes. The sample was then centrifuged at 2330 g for 25 minutes to sediment the resulting lysate cell and platelet debris. To confirm the absence of platelets, an aliquot of 200 µL was measured in an impedance hemacytometer (Diagno-Icounter vet®). The lysis technique was adapted from studies by Gilbertie, Perrone, and Hagen [16-18].
Aliquoting and storage of blood products
The products were organized and labeled in groups, animals, treatments, evaluation days, and temperature. Seven animals, two treatments (AS and PL), three days of evaluation over an eight days period (0, 4, 8) and two temperatures of 4 and 37 °C.
The products were aliquoted into 24 sterile vials. Twelve vials of 1.5 mL (ELISA, TGF-β1), and twelve of 0.5 mL (PROTEOMIC), per animal, for (AS and PL), at times: 0, 4 and 8 days for the two evaluation temperatures.
Each sample was placed at 4 °C and 37 °C, remaining there for the designated storage, temperatures, and times intervals, in addition, they were also preserved at -80 °C for ELISA kit protein and growth factor analysis.
Measurement of total protein and albumin
A random-access analyzer for veterinary biochemistry (SMART 200 VET, Biotécnica, epimed, Porto Alegre, Brazil) was used to test total protein (PTT) and albumin (ALB). The samples were removed from their -80 °C frozen storage and left to thaw for 20 minutes before processing. 100 µL of serum and plasma were deposited in each cuvette of the equipment for their respective evaluation. It took several times to process the 84 samples; the equipment can analyze 18 samples per time. The results obtained by the equipment were processed automatically through computer software, obtaining results for the two analytes expressed in g/dL.
Measurement of TGF-β1
A commercial TGF-1 ELISA Kit (Enzyme-Linked Immunosorbent Assay for Quantitative Detection of Human TGF-1,2019) was used to examine the TGF-1 growth factor present in autologous serum and platelet lysate kit validated for the equine specie in 92% [11] and 98% [13].
Four animals were chosen at random to do the ELISA test, which was performed in duplicate. The growth factor of each of the four animals was measured, for both groups AS and PL, at the three storage times (0, 4 and 8 days) and the two temperatures of 4 °C and 37 °C. The samples were defrosted at room temperature on the day of processing, and the manufacturer’s instructions were followed.
Statistical analysis
All static analyzes were performed using the R software version 3.6.1 (R Core Team, 2019). The data were used for a descriptive study of the animal profiles. All factors were evaluated in this descriptive analysis. Calculations were made for the primary descriptive statistics indices (mean, median, standard deviation, coefficient of variation, and quartiles). This analysis was performed for all animals, as well as separately for pre-defined groups to provide a specific profile, in the following variables platelets, erythrocytes, leukocytes, growth factors, and proteins. To evaluate the differences between products, times, and temperatures, linear regression models were fitted. The different products, times, and temperatures were used as predictor variables. It was necessary to apply the BoxCox transformation to the response variable to control the variance and reduce the impact of outliers. After adjusting the model, the mean values and their respective 95% confidence intervals were calculated. Multiple comparison tests (pairwise) were performed by applying the Sidak correction. For all tests, a significance level of 5% was used.
Characteristics of the obtained products
During the product evaluation, an average platelet concentration in platelet-rich plasma of 366,000 platelets per microliter was found, which corresponds to 2.59 times the basal platelet concentration. The other means were initial platelet concentration of 140,857/µL, 6,431 leukocytes/µL, and 7.2 x 10^6 erythrocytes/µL Table 1.
| Table 1: Figurelet,leukocyte, and erythrocyte concentrations in the whole blood of each animal,as well as the platelet concentration after processing with platelet-richplasma (PRP). Additionally, the number of times each animal was able toachieve platelet concentration. Demonstrating the protocol's efficacy. | |||||
| Animals | Figureles / µL | Leukocytes / µL | Erythrocyte X | PRP Figurelets/ µL | Times concentration |
| 178.000 | 4.700 | 6,76 | 548.000 | 3.0 x | |
| 126.000 | 1.500 | 7,4 | 303.500 | 2.4 x | |
| 86.000 | 10.900 | 7,52 | 251.000 | 2.9 x | |
| 196.000 | 6.700 | 7,66 | 457.000 | 2.3 x | |
| 90.000 | 9.300 | 6,67 | 270.000 | 3.0 x | |
| 126.000 | 5.800 | 7,45 | 267.000 | 2.1 x | |
| 184.000 | 6.120 | 6,93 | 464.000 | 2.5 x | |
| Mean | 140.857 | 6.431 | 7,2 | 366.000 | 2.59 x |
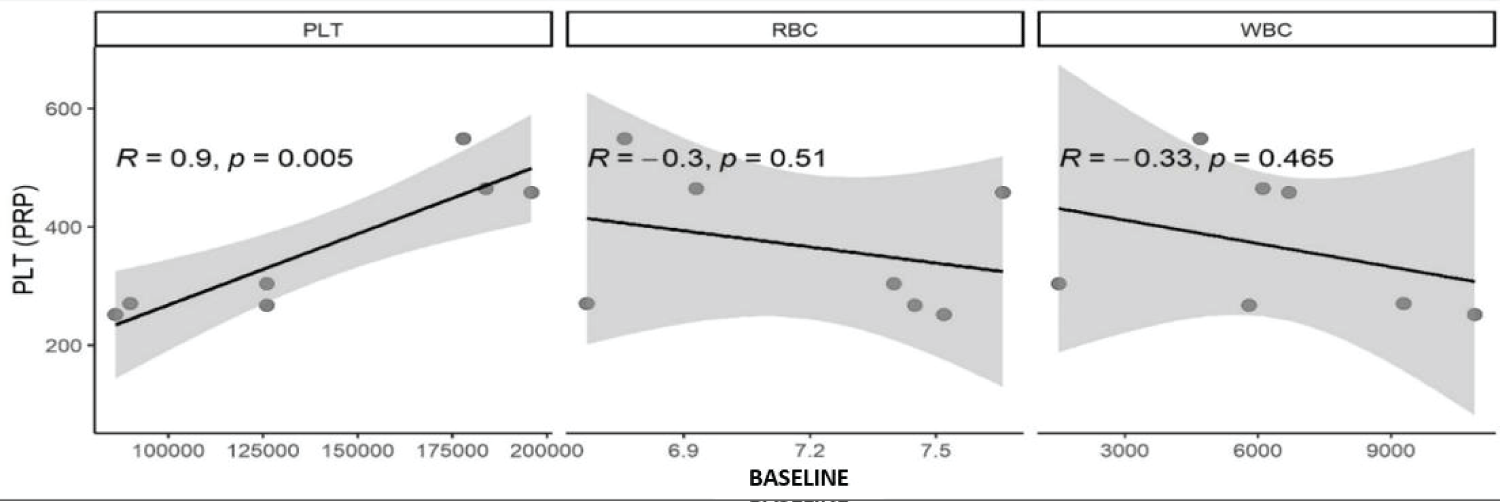
A statistically significant correlation was found between the basal platelet concentration and the platelet concentration of the PRP produced (p < 0.005) in the analysis of the hematological parameters of the animals and blood products Figure 1.
Figure 1: Scatter plots, where (R) is Spearman's correlation value and P is the correlation value. Statistical correlation in cell numbers of platelets (PLT), leukocytes (WBC), and erythrocytes (RBC) concentrations in PRP compared with values of the same cells in whole blood.
The lysis protocol performed was adequate to produce a platelet lysate, a total of 3 repetitive freezing and thawing cycles was required to reach the PL with a platelet count of 0.
On the other hand, the presence of a minimal number of leukocytes and red blood cells was detected.
Total protein and albumin in PL and AS
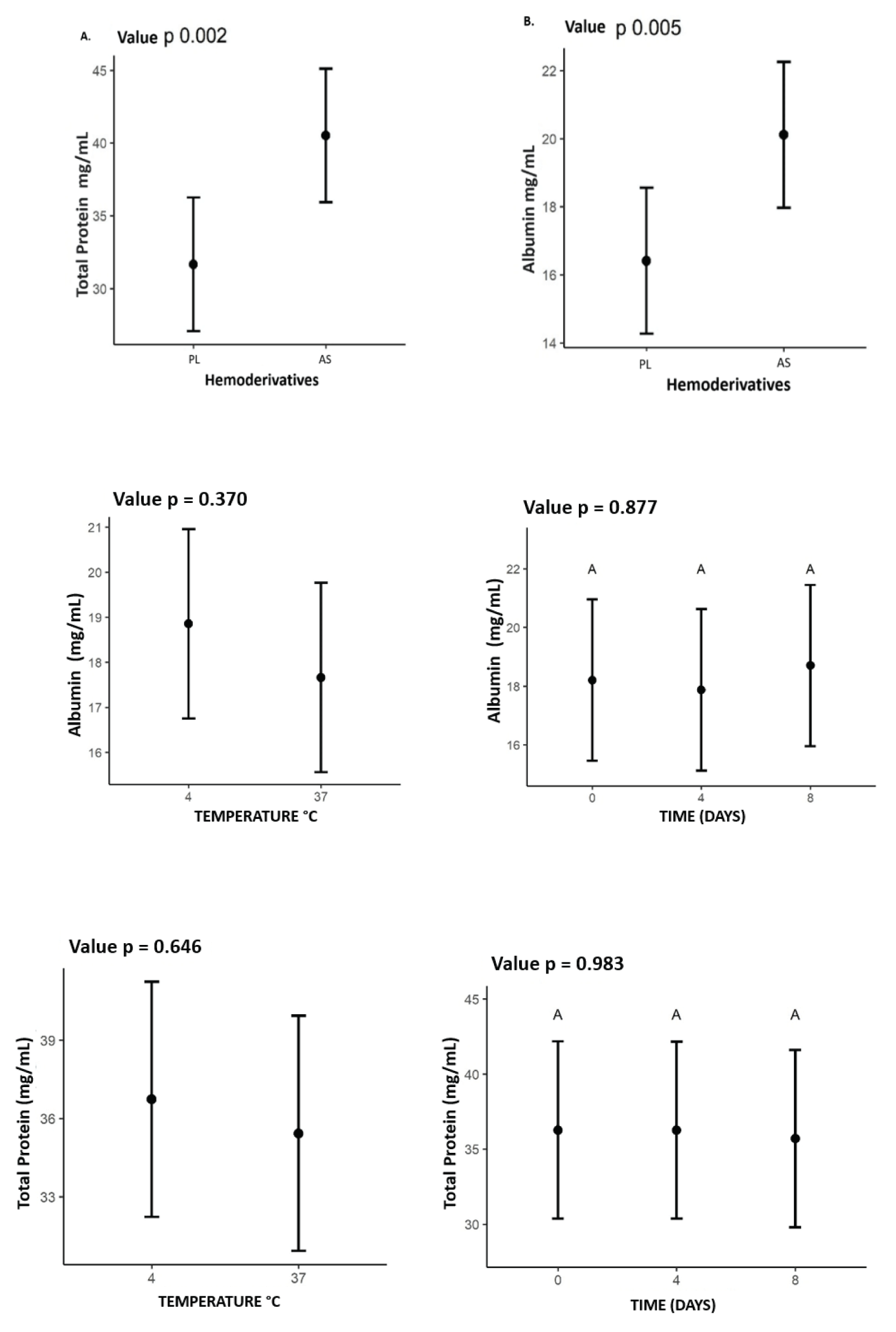
Total protein and albumin data were obtained on average for the 7 animals, at the three storage times and at the two temperatures for each product. The mean total protein in the PL was 31.65 mg/mL and in the autologous serum, it was 40.51 mg/mL. Albumin for the PL showed an average of 16.41 mg/mL and for the serum 20.11 mg/mL. AS showed a higher concentration for both parameters (p < 0.05) (Figure 2).
Figure 2: Mean values of total protein and albumin expressed (mg/mL) for each of the products, PL and AS. Showing higher concentration in SA for the two proteins, with value for the total protein of A. p = 0.002 and B. albumin p = 0.005, also the values for Albumin and total protein relating the influence of time (0.4.8 days) and temperature (4°C) and (37°C) on concentrations in PL and AS.
There was no statistics difference between total protein and albumin concentrations concerning temperature or storage time as illustrated in Figure 2.
TGF-β1 growth factor
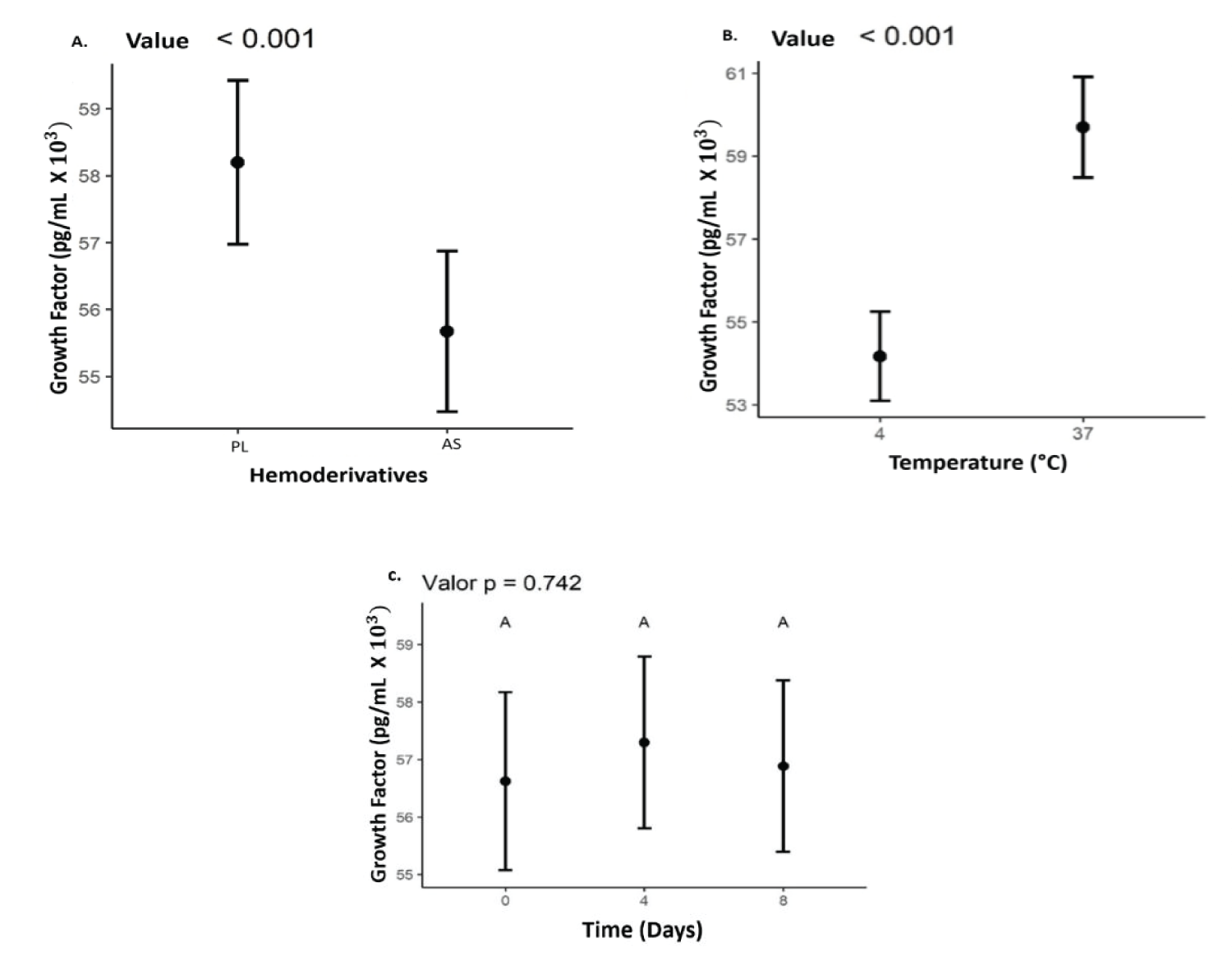
The mean values found for TGF-β1 for PL and AS were 5,940 pg/mL and 5,530 pg/mL, respectively, including all times and temperatures. The concentration of TGF-β1 was higher (p < 0.05) in the PL. A higher concentration of these GFs was also observed at a temperature of 37 °C (p < 0.05). However, there was no difference in GFs concentration when compared to storage times Figure 3.
Figure 3: Values representing the concentration of the growth factor TGF-β1 values expressed in (pg/mLx10^3), (B) Temperatures (4°C and 37°C); (C) storage time (0, 4, 8) days in (A) AS and PL.
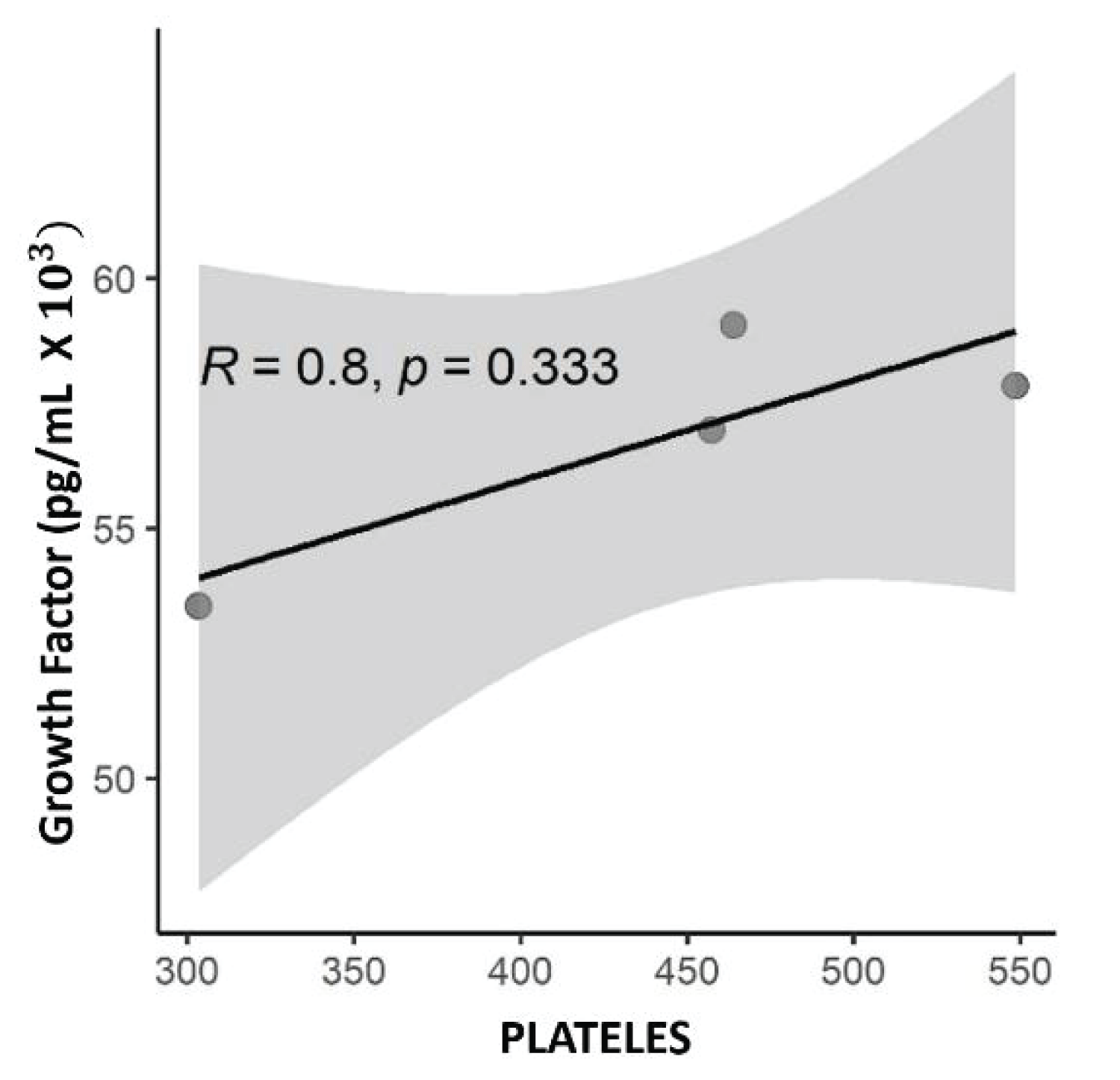
The correlation between TGF-β1 concentration and baseline PRP platelet count, acquired before platelet processing and lysis, was tested. There was a strong correlation (R = 0.8), although not significant (p = 0.333) (Figure 4).
Figure 4: Scatter plot with numerical values, with the correlation value R= 0.8 of Spearman and p, the value p = 0.333 being significant for this correlation. Correlation between platelet concentration and TGF-β1 growth factor concentration expressed in pg/mL.
Although there are several definitions for considering a blood product as PRP. In the present study, it is considered that the protocol used was adequate to produce a PRP. Using the modified double-centrifugation methodology, an average concentration of 2.59 times compared to the basal level was reached, which could achieve adequate conditions for the use of this therapy. Smith [19], in his in vitro study on the anabolic effect of PRP in an equine tendon, concluded that a concentration of 4 times the basal concentration would be necessary to observe a reparative effect. These data are compatible with the one proposed by Marx [20], which states that a cellular repair response was evident when the product had concentrations of 4 to 5 times the number of basal platelets. However, other studies consider lower concentrations as positive. Anitua [21] and Carmona [22] recommend a count of 300,000 platelets/µl, which represents 1-2.5 times the baseline platelets of whole blood. Vendruscolo [23] proposes 350,000 platelets/µL, ie 2.6 times the baseline.
Hematological parameters also influence platelet concentration in PRP. The platelet concentration in the final PRP showed a positive correlation (R = 0.9; p = 0.005) with the initial platelet concentration, which is in line with the literature [24].
The PRP lysis protocol used in the present study was adequate to eliminate platelets in all samples after 3 cycles and final centrifugation. On the other hand, as observed in Table 2, a mean different from 0 was obtained for leukocytes and red blood cells. Possibly, this observation is related to the impedance counting technique, in which small cell fragments can be counted as cells. Rushton [25], using the impedance technique, found high amounts of protein precipitates of various sizes, as well as cellular debris, which were counted as cells, due to their size being identical to white blood cells. This unreliable count is even more likely when the number of cells is low [26]. Although other platelet counting techniques have been identified as more accurate, both manual counting and flow cytometry have limiting factors. Manual counting is a time-consuming and laborious technique, which makes its applicability in a large volume of samples difficult. On the other hand, flow cytometry has a very high cost, which limits its use in many scenarios. For these reasons, impedance has been widely accepted in PRP studies [27-31].
| Table 2: Mean values of platelets, erythrocytes, and leukocytes per microliter after the LP protocol, performed for 3 cycles of freezing (-80°C), for 30 minutes in µL trafrezzer, and thawing, at (37°C) for 10 minutes in a water bath, performed in the equine species. |
|||
| Hemoderivate LP | Figurelets / µL | Erythrocyte / µL | Leukocytes / µL |
| Media | 0,000 | 0,041 | 0,049 |
After the lysis process, two techniques have been used to reduce the presence of cell fragments: centrifugation and filtration [18,32,33]. In the present study, we chose to perform centrifugation without the use of a filter, as a way of evaluating the lysis method within a scenario of routine use, in which the filter is often not available. In addition, the product produced was not intended for in vivo use, therefore, there is no concern from an immunogenic point of view.
To monitor possible influences of the parameters studied on the composition of blood products, the concentration of total proteins and albumin in the products obtained was measured. It was observed that the concentrations were higher for both analytes in the SA. Hagen [18] in his study of lysed plasma in horses, from a platelet concentrate, measured and identified the composition of different blood products to characterize the products. In this work, the concentration of total protein in the PL was (53.57 ± 2.08 mg/mL) and albumin (27.99 ± 1.10 mg/mL); in autologous serum, they obtained a total protein of (59.69 ±2.25 mg/mL) and albumin (32.26 ±1.44 mg/L). These results are compatible with this research, in terms that both total protein and albumin in the AS are higher compared to the concentrations in the PL. On the other hand, it is necessary to clarify that these findings are unusual if compared with studies of protein profiles in the equine species for serum and plasma. In theory, due to the processes by which serum and plasma are obtained, the literature shows that serum has a lower concentration of proteins concerning plasma, under conventional processing and storage conditions. This is explained during the clotting process, where the serum becomes qualitatively different from the plasma. The removal of a large concentration of fibrinogen from the blood plasma, in the formation of the fibrin clot, results in the serum from this process having a lower protein concentration than the plasma, due to the loss of this protein [34]. The fact that higher protein concentrations were found in the AS compared to the PL in the present study, and in studies comparing the same products as the one carried out by Hagen [18], may be related to the processes of obtaining and lysing PRP. It can be inferred that the repetitive cycles of centrifugation, as well as freezing and thawing with drastic temperatures, could cause the proteins to decrease since the AS did not go through these processes, but the two products were stored in the same conditions, time and temperature, and these variables have not been shown to make or have a statistical difference between total protein or albumin concentration.
The measurement and estimation of growth factors in blood products, even being extremely important to quantify precise therapeutic concentrations, can’t be used routinely, due to high costs and the need for experienced personnel for its evaluation. Within eye drops, the measurement of epitheliotropic growth factors is one of the most important processes, but little is performed [15].
In the present study, TGF-β1 ELISA was performed, finding results on average for the PL of (5,940 pg/mL), and for the AS of (5,530 pg/mL). These results are consistent with other studies, in terms of the difference that exists between the concentration of both products. On the other hand, in this study, the concentrations obtained were higher in both AS and PL compared to the results of other studies. Giraldo [35] found from the lysate of a P-PRP, with concentrations of (304,030 platelets/µL), TGF-β1 concentration of (3192.7 ± 1395.3), in comparison with the findings of this work, were lower values. The above may be related to the fact that the P-PRP obtained in this study had a lower platelet concentration than that achieved in the present study (366,000 platelets/µL), consequently lower GF release. The above is more evident in the study in horses by Textor [36] which, through a protocol of 3 centrifugations, concentrated 1´000,000 platelets/µL, and after the lysate with ionic detergent, he obtained concentrations of TGF-β1 in the range of 22,677 ± 12.125 pg/ml.
In autologous serum the concentration of TGF-β1 was lower compared to PL, showing a reliable statistical significance level of p < 0.001. The finding in the present study is broadly related to and supported by the literature. In AS, the process of releasing growth factors takes place through the process of natural clotting of whole blood, this process is carried out by autologous thrombin, thus inducing degranulation and release of growth factors present in the basal blood [37,38]. On the other hand, it can be inferred that the concentration of TGF-β1 in the AS was lower since there is no platelet concentration higher than in the blood. Furthermore, according to Textor and Tablin [39] activation by equine autologous thrombin, in the natural process of coagulation, sequesters growth factors, which are kept within this membrane, substantially decreasing the concentrations of GFs in the resulting serum. As demonstrated by Burnouf [38] in human medicine where GFs in serum decreased between 30% and 50% compared to PL.
In this work, it seems that both in the AS and in the PL, the GFs had a higher concentration, at the storage temperature of 37 °C. The literature does not present many studies on these findings. This temperature was considered as a storage group, to verify if it was related to a higher concentration, and release of growth factors. Many studies show that activation methodologies at 37 °C obtain good levels of growth factors [40,41]. There are still no studies on the storage of lysates at 37 °C, which show if there is any influence on the release or stability of these GFs. On the other hand, due to the lack of studies on PL storage, it was not possible to compare the stability of this blood product over time. In this study, there was no statistically significant increase or decrease in the growth factor during the 8 days of storage p = 0.737.
TGF-β1 may have anabolic and catabolic properties in eye tissue. The literature does not present a definitive adequate concentration to optimize its positive effects [42]. In the present study, both blood products reached relatively high concentrations of TGF-β1, compared with similar products in the literature, such as those found by Haber [43], in this work they showed that TGF-β1 at concentrations of 1,100 pg/mL and 2,000 pg/ mL significantly decreased epithelial cell proliferation compared to controls; in the case of lower concentrations, 500 pg/ml, cell proliferation did not decrease, and cell growth was stable.
Regarding the relationship between the number of platelets and the release of growth factors, not the case of TGF-β1, the literature remains controversial. No present work was possible to find a strong correlation between platelets and TGF-β1 (R = 0.8), this result is statically defined, as a strong correlation after the value is closer to (R = 1). The value of (p = 0.333), is not significant, therefore, some factors can intervene in this result such as the sample size [44]. These results not present in this study can be compared to those reported by Sundman [14] where the release and concentration of TGF-β1 would be dependent on the cellular composition of PRP. To compare two PRPs, one of them with low platelet concentration and the other with high concentration. In high concentrations, there was a strong correlation between TGF-β1 and platelets R = 0.75 and p = 0.001, also corroborating the hypothesis of their study. Hagen [18] studying an equine species found a moderate to strong association of TGF-β1 in a platelet concentrate. The evaluation was made on a plasma lysate obtained by repetitive cycles of freezing and thawing and the relationship of TGF-β1 to platelet lysate was R = 0.626 p = 0.01 showing a good statistical correlation, in addition to the reliability of the two data. The former explains it theoretically, once a product with a high platelet concentration would have a greater capacity to release growth factors since platelets are an important autologous source of these factors. Release occurs through platelet intracellular signaling pathways, through interaction with a complex network of surface receptors on other cells and tissues [14,23,45].
The choice between using autologous serum and platelet lysate for therapeutic purposes can be a complex decision and may depend on various factors, including the specific therapeutic application, availability, cost, and preparation. Studies have shown that autologous serum can promote cell proliferation, migration, and differentiation, making it a potential therapeutic option [8]. Figurelet lysate, on the other hand, requires more complex preparation steps. It involves collecting whole blood from the patient, isolating platelets, and then lysing them to release growth factors and other bioactive molecules [38]. In terms of growth factor concentration, platelet lysate has been shown to have higher levels of growth factors than autologous serum. Figurelet lysate may be a better choice for more severe ocular surface disorders or corneal wounds that require a higher concentration of growth factors to promote tissue repair and regeneration.
The basal value of platelets in the blood and the platelet concentration in the final platelet-rich plasma (PRP) are strongly correlated.
The concentration of TGF- β1 is higher in PL when compared to AS. However, the reason for this disparity is yet to be determined by this study. Notably, the outcomes demonstrate that TGF- β1 levels were most abundant at a temperature of 37 °C for both AS and PL.
In comparison to platelet lysate, traditional autologous serum showed a higher concentration of total protein and albumin. Their concentrations were unaffected by time or temperature in this study.
The choice between using autologous serum and platelet lysate depends on various factors, including the specific clinical application, availability, and cost. Both autologous serum and platelet lysate have potential therapeutic benefits and can promote tissue repair and regeneration through different mechanisms. Therefore, it is important to consider the specific needs of each animal or patient before taking a decision.
Data availability
The data used to support the findings of this study were supplied by [Camilo Osorio Florez]. Requests for access to these data should be made to [Camilo Osorio Florez, [email protected], website https://www.researchgate.net/profile/Camilo-Osorio-Florez]
- Maia L, De Souza MV. Components rich in platelets used in wound healing tendon, ligaments and osteo-articular diseases of animals. Ciência Rural. 2009; 39: 1279–1286.
- De Souza MV, Pinto O, Da Costa MM. Quantification of growth factors in horse skin treated with platelet-rich plasma. 2014; 34: 599–612.
- Mirza AUB, Ghani N, Khan AB. Epitheliotrophic Effect of Autologous Serum in Persistent Corneal Epithelial Defects. Pakistan Journal of Ophthalmology. 2008; 24:19-26.
- López-García JS, García-Lozano I, Rivas L, Martínez-Garchitorena J. Aplicaciones del suero autólogo en oftalmología [Use of autologous serum in ophthalmic practice]. Arch Soc Esp Oftalmol. 2007 Jan;82(1):9-20. Spanish. doi: 10.4321/s0365-66912007000100004. PMID: 17262232.
- Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017 Feb 28;2(2):CD009327. doi: 10.1002/14651858.CD009327.pub3. PMID: 28245347; PMCID: PMC5510593.
- Mandelbaum SH, Di Santi, É, Mandelbaum M. Cicatrization: current concepts and auxiliary resources - Part II. An Bras Dermatol. 2003; 78: 525–542.
- Paganela JC, Ribas LM, Santos CA. Abordagem clínica de feridas cutâneas em equinos Clinical approach in equine skin wounds. Revista Portuguesa de Ciências Veterinárias. 2009; 104: 569–572.
- Anitua E, Muruzabal F, Tayebba A, Riestra A, Perez VL, Merayo-Lloves J, Orive G. Autologous serum and plasma rich in growth factors in ophthalmology: preclinical and clinical studies. Acta Ophthalmol. 2015 Dec;93(8):e605-14. doi: 10.1111/aos.12710. Epub 2015 Apr 2. PMID: 25832910.
- Schallmoser K, Henschler R, Gabriel C, Koh MBC, Burnouf T. Production and Quality Requirements of Human Figurelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2020 Jan;38(1):13-23. doi: 10.1016/j.tibtech.2019.06.002. Epub 2019 Jul 17. PMID: 31326128.
- Soares CS, Babo PS, Reis RL, Carvalho PP, Gomes ME. Figurelet-Derived Products in Veterinary Medicine: A New Trend or an Effective Therapy? Trends Biotechnol. 2021 Mar;39(3):225-243. doi: 10.1016/j.tibtech.2020.07.011. Epub 2020 Aug 28. PMID: 32868100.
- Penha-Goncalves MN, Onions DE, Nicolson L. Cloning and sequencing of equine transforming growth factor-beta 1 (TGF beta-1) cDNA. DNA Seq. 1997;7(6):375-8. doi: 10.3109/10425179709034059. PMID: 9524819.
- Argüelles D, Carmona JU, Pastor J, Iborra A, Viñals L, Martínez P, Bach E, Prades M. Evaluation of single and double centrifugation tube methods for concentrating equine platelets. Res Vet Sci. 2006 Oct;81(2):237-45. doi: 10.1016/j.rvsc.2005.12.008. PMID: 16969921.
- Watts EJ, Rose MT. Figurelet-derived growth factor acts via both the Rho-kinase and p38 signaling enzymes to stimulate contraction in an in vitro model of equine wound healing. Domest Anim Endocrinol. 2010 May;38(4):253-9. doi: 10.1016/j.domaniend.2009.11.004. Epub 2009 Dec 6. PMID: 20036481.
- Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011 Oct;39(10):2135-40. doi: 10.1177/0363546511417792. Epub 2011 Aug 16. PMID: 21846925.
- Sanak F, Baenninger P, Kaufmann C, Iselin K, Bachmann L, Buhl D, Thiel M. The Lucerne Protocol for the Production of Autologous Serum Eyedrops. Klin Monbl Augenheilkd. 2021 Apr;238(4):346-348. English. doi: 10.1055/a-1354-6565. Epub 2021 Apr 30. PMID: 33930907.
- Gilbertie JM, Schaer TP, Schubert AG, Jacob ME, Menegatti S, Ashton Lavoie R, Schnabel LV. Figurelet-rich plasma lysate displays antibiofilm properties and restores antimicrobial activity against synovial fluid biofilms in vitro. J Orthop Res. 2020 Jun;38(6):1365-1374. doi: 10.1002/jor.24584. Epub 2020 Jan 14. PMID: 31922274; PMCID: PMC8018705.
- Perrone G, Lastra Y, González C, Caggiano N, Giménez R, Pareja R, De Simone E. Treatment With Figurelet Lysate Inhibits Proteases of Synovial Fluid in Equines With Osteoarthritis. J Equine Vet Sci. 2020 May;88:102952. doi: 10.1016/j.jevs.2020.102952. Epub 2020 Feb 1. PMID: 32303304.
- Hagen A, Lehmann H, Aurich S, Bauer N, Melzer M, Moellerberndt J, Patané V, Schnabel CL, Burk J. Scalable Production of Equine Figurelet Lysate for Multipotent Mesenchymal Stromal Cell Culture. Front Bioeng Biotechnol. 2021 Jan 21;8:613621. doi: 10.3389/fbioe.2020.613621. PMID: 33553119; PMCID: PMC7859354.
- Smith JJ, Ross MW, Smith RK. Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibroblasts in vitro. Vet Comp Orthop Traumatol. 2006;19(1):43-7. PMID: 16594543.
- Marx RE. Figurelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004 Apr;62(4):489-96. doi: 10.1016/j.joms.2003.12.003. PMID: 15085519.
- Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004 Jan;91(1):4-15. doi: 10.1160/TH03-07-0440. PMID: 14691563.
- Carmona JU, López C, Giraldo CE. Use of autologous platelet concentrates as regenerative therapy for chronic diseases of the equine musculoskeletal system. Archivos de Medicina Veterinaria. 2011; 43:1-10.
- Vendruscolo CP, Carvalho A, Moraes LF. Evaluating the effectiveness of different protocols for preparation of platelet rich plasma for use in equine medicine. Pesquisa Veterinaria Brasileira. 2012; 32: 106-110.
- Andrade MG, de Freitas Brandão CJ, Sá CN, de Bittencourt TC, Sadigursky M. Evaluation of factors that can modify platelet-rich plasma properties. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Jan;105(1):e5-e12. doi: 10.1016/j.tripleo.2007.07.032. PMID: 18155603.
- Rushton JO, Kammergruber E, Tichy A, Egerbacher M, Nell B, Gabner S. Effects of three blood derived products on equine corneal cells, an in vitro study. Equine Vet J. 2018 May;50(3):356-362. doi: 10.1111/evj.12770. Epub 2017 Nov 3. PMID: 29044680.
- Springer W, von Ruecker A, Dickerhoff R. Difficulties in determining prophylactic transfusion thresholds of platelets in leukemia patients. Blood. 1998 Sep 15;92(6):2183-4. PMID: 9731080.
- Álvarez ME, López C, Giraldo CE. In-vitro bactericidal activity of equine platelet concentrates, platelet poor plasma, and plasma against methicillin-resistant Staphylococcus aureus. Archivos de Medicina Veterinaria. 2011; 43: 155–161.
- Anitua E, Alonso R, Girbau C, Aguirre JJ, Muruzabal F, Orive G. Antibacterial effect of plasma rich in growth factors (PRGF®-Endoret®) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol. 2012 Aug;37(6):652-7. doi: 10.1111/j.1365-2230.2011.04303.x. Epub 2012 Feb 14. PMID: 22329713.
- Drago L, Bortolin M, Vassena C, Taschieri S, Del Fabbro M. Antimicrobial activity of pure platelet-rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013 Feb 25;13:47. doi: 10.1186/1471-2180-13-47. PMID: 23442413; PMCID: PMC3599521.
- Drago L, Bortolin M, Vassena C, Romanò CL, Taschieri S, Del Fabbro M. Plasma components and platelet activation are essential for the antimicrobial properties of autologous platelet-rich plasma: an in vitro study. PLoS One. 2014 Sep 18;9(9):e107813. doi: 10.1371/journal.pone.0107813. PMID: 25232963; PMCID: PMC4169456.
- López C, Alvarez ME, Carmona JU. Temporal Bacteriostatic Effect and Growth Factor Loss in Equine Figurelet Components and Plasma Cultured with Methicillin-Sensitive and Methicillin-Resistant Staphylococcus aureus: A Comparative In Vitro Study. Vet Med Int. 2014;2014:525826. doi: 10.1155/2014/525826. Epub 2014 Nov 24. PMID: 25506468; PMCID: PMC4260436.
- Bernardi M, Albiero E, Alghisi A, Chieregato K, Lievore C, Madeo D, Rodeghiero F, Astori G. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2013 Aug;15(8):920-9. doi: 10.1016/j.jcyt.2013.01.219. Epub 2013 Apr 24. PMID: 23623274.
- Gilbertie JM, Long JM, Schubert AG, Berglund AK, Schaer TP, Schnabel LV. Pooled Figurelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes From Synoviocyte-Derived Inflammatory Mediators. Front Vet Sci. 2018 Jul 4;5:150. doi: 10.3389/fvets.2018.00150. PMID: 30023361; PMCID: PMC6039577.
- Issaq HJ, Xiao Z, Veenstra TD. Serum and plasma proteomics. Chem Rev. 2007 Aug;107(8):3601-20. doi: 10.1021/cr068287r. Epub 2007 Jul 18. PMID: 17636887.
- Giraldo CE, López C, Álvarez ME, Samudio IJ, Prades M, Carmona JU. Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet Res. 2013 Feb 12;9:29. doi: 10.1186/1746-6148-9-29. PMID: 23402541; PMCID: PMC3577464.
- Textor JA, Norris JW, Tablin F. Effects of preparation method, shear force, and exposure to collagen on release of growth factors from equine platelet-rich plasma. Am J Vet Res. 2011 Feb;72(2):271-8. doi: 10.2460/ajvr.72.2.271. PMID: 21281204.
- Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009 Sep;27(9):2331-41. doi: 10.1002/stem.139. PMID: 19544413.
- Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016 Jan;76:371-87. doi: 10.1016/j.biomaterials.2015.10.065. Epub 2015 Oct 28. PMID: 26561934.
- Textor JA, Tablin F. Activation of equine platelet-rich plasma: comparison of methods and characterization of equine autologous thrombin. Vet Surg. 2012 Oct;41(7):784-94. doi: 10.1111/j.1532-950X.2012.01016.x. Epub 2012 Jun 28. PMID: 22742830.
- Gutiérrez CM, López C, Giraldo CE, Carmona JU. Study of a Two-Step Centrifugation Protocol for Concentrating Cells and Growth Factors in Bovine Figurelet-Rich Plasma. Vet Med Int. 2017;2017:1950401. doi: 10.1155/2017/1950401. Epub 2017 Oct 30. PMID: 29214094; PMCID: PMC5682892.
- Steller D, Herbst N, Pries R, Juhl D, Hakim SG. Impact of incubation method on the release of growth factors in non-Ca2+-activated PRP, Ca2+-activated PRP, PRF and A-PRF. J Craniomaxillofac Surg. 2019 Feb;47(2):365-372. doi: 10.1016/j.jcms.2018.10.017. Epub 2018 Nov 15. PMID: 30578012.
- Donnelly KS, Giuliano EA, Sharm A, Mohan RR. Suberoylanilide hydroxamic acid (vorinostat): its role on equine corneal fibrosis and matrix metalloproteinase activity. Vet Ophthalmol. 2014 Jul;17 Suppl 1:61-8. doi: 10.1111/vop.12129. PMID: 25126665.
- Haber M, Cao Z, Panjwani N, Bedenice D, Li WW, Provost PJ. Effects of growth factors (EGF, PDGF-BB and TGF-beta 1) on cultured equine epithelial cells and keratocytes: implications for wound healing. Vet Ophthalmol. 2003 Sep;6(3):211-7. doi: 10.1046/j.1463-5224.2003.00296.x. PMID: 12950652.
- Arias MM. What does the p-value really mean? Pediatric Primary Care. 2017; 19: 377–381.
- Anitua E, Andía I, Sanchez M, Azofra J, del Mar Zalduendo M, de la Fuente M, Nurden P, Nurden AT. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005 Mar;23(2):281-6. doi: 10.1016/j.orthres.2004.08.015. PMID: 15779147.