Abstract
Research Article
Evaluation of Single Bilateral Intratesticular Injection of Cetrimide for Nonsurgical Sterilization of Adult Male Albino Mice
Haben Fesseha* and Guesh Negash
Published: 27 July, 2020 | Volume 4 - Issue 1 | Pages: 025-034
Nonsurgical fertility control is increasingly advocated as more cost-effective than surgical sterilization to manage stray animal populations in a different part of the world. An experimental study was conducted from December 2018 to April 2019 at Mekelle University to evaluate the effect of single bilateral intratesticular injection of cetrimide 2% in adult albino mice. A total of 20 clinically healthy albino mice selected based on their age and sex and were divided randomly into five groups and evaluation was conducted for 30 days after intratesticular injection of cetrimide solution 2% at the dose rate of 5, 10, 15 and 20 mg per testis and for control 0.1 mL normal saline per testis per 100 g body weight were given. All albino mice were evaluated for 30 days at a fixed interval. Change in body weight, scrotal width, sexual behavior, and fertility performance was also assessed. On day 30, all albino mice were sacrificed for histopathological study. Means ± Standard deviation of the mean, one-way, and a mixed model ANOVA (for repeated measures) was used to summarize the data, determine the effects of group and time on bodyweight and scrotal width. The significant increase in body weight (p - 0.001) and significant reduction of scrotal width (p - 0.001) were noted in all cetrimide treated in comparison to control groups. In addition, there was a significant (p < 0.05) reduction of scrotal width in albino mice after intratesticular injection of cetrimide on day 1, 10, 15, 20, 25, and 30 with respect to their experimental groups. Testicular histology revealed that there were multinucleated giant cells in seminiferous tubules, derangement of tubular architecture along with infiltration of leucocytes and appearance of fibrous tissue were seen on testicular sections at a dose rate of 15 and 20 mg. Similarly, a significant change in the sexual behavior of the treated males and no pregnancy was detected on female albino mice after 21 days post-coital at 10, 15, or 20 mg cetrimide-treated males. In conclusion, a single bilateral intratesticular injection of cetrimide 2% at a dose of 15 and 20 mg might provide an effective way of sterilization and may be considered as an alternative to surgical castration in male animals. Besides, further assessment should be done in the future to identify the mechanism of infertility.
Read Full Article HTML DOI: 10.29328/journal.ivs.1001023 Cite this Article Read Full Article PDF
Keywords:
Albino mice; Cetrimide; Histopathology; Testicle; Chemical sterilization; Intratesticular injection
References
- Auer JA, Stick J. Reproductive system in equine surgery. 3rd ed. Equine surgery, ed. In: Auer JA, editors. . 2006, USA: Saunders Elsevier. 282-295.
- Ibrahim A. Evaluation of chemical castration with calcium chloride versus surgical castration in donkeys: testosterone as an endpoint marker. BMC Vet Res. 2016; 12: 46.
- Jana K, Samanta PK. Sterilization of male stray dogs with a single intratesticular injection of calcium chloride: a dose-dependent study. Contraception. 2007; 75: 390-400. PubMed: https://pubmed.ncbi.nlm.nih.gov/17434022/
- Fesseha H. Non-surgical sterilization methods in male animals: A review. Vet Med Open J. 2019; 4: 49-56.
- Leoci R, Aiudi G, Silvestre F, Lissner EA, Marino F, et al. A dose-finding, long-term study on the use of calcium chloride in saline solution as a method of nonsurgical sterilization in dogs: evaluation of the most effective concentration with the lowest risk. Acta Vet Scand. 2014; 56: 63. PubMed: https://pubmed.ncbi.nlm.nih.gov/25317740
- Matsumoto AM. Is high dosage testosterone an effective male contraceptive agent? Fertility and sterility, 1988. 50: 324-328. PubMed: https://pubmed.ncbi.nlm.nih.gov/3396702/
- Swerdloff R, Wang C, Bhasin S. 10 Developments in the control of testicular function. Bailliere's clinical endocrinology and metabolism. 1992; 6: 451-483. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4801682/
- Dube D, Assaf A, Pelletier G, Labrie F. Morphological study of the effects of an GnRH agonist on the canine testis after four months of treatment and recovery. Eur J Endocrinol, 1987; 116: 413-417.
- Wu FC, Aitken RJ. Suppression of sperm function by depot medroxyprogesterone acetate and testosterone enanthate in steroid male contraception. Fertil Sterility. 1989; 51: 691-698.
- Tremblay Y, Bélanger A. Reversible inhibition of gonadal functions by a potent gonadotropin-releasing hormone agonist in adult dog. Contraception. 1984; 30: 483-497.
- Gonzalez A. Immunological approaches to contraception in dogs. J Reproduct Ferti Suppl. 1989; 39: 189-198.
- Leoci R, Aiudi G, Silvestre F, Lissner EA, Lacalandra GM. Alcohol diluent provides the optimal formulation for calcium chloride non-surgical sterilization in dogs. Acta Vet Scand. 2014; 56: 62.
- Kar AB, Kamboj V, Goswami A. Sterilization of male rhesus monkeys by iron salts. Reproduction. 1965; 9: 115-117.
- Gebremeskel H. Chemosterilization Effct of Single Intratesticular Injection of Chlorhexidine in Male Adult Albino Mice-A Histopathological Study. J Tissue Biol Cytol. 2020; 3: 005.
- Emir L. Hormonal and pathologic changes after chemoablation of testes with hypertonic saline solution as a treatment method alternative to orchiectomy in patients with hormone sensitive metastatic prostatic cancer. Urologic Oncology: Seminars and Original Investigations. 2011; 29: 212-217.
- Jana K, Samanta PK, Ghosh D. Dose-dependent response to an intratesticular injection of calcium chloride for induction of chemosterilization in adult albino rats. Vet Res Community. 2002; 26: 651–673. PubMed:
- Jana K, Samanta PK. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception. 2006; 73: 289-300. PubMed: https://pubmed.ncbi.nlm.nih.gov/16472573/
- Nishimura N, Kawate N, Sawada T. Chemical castration by a single intratesticular injection of lactic acid in rats and dogs. Journal of Reproduction and Development. 1992; 38: 263-266. PubMed:
- Oliveira EC, Moura MRP, de Sá MJC, Silva Jr VA, Kastelic JP, et al. Permanent contraception of dogs induced with intratesticular injection of a zinc gluconate-based solution. Theriogenology. 2012; 77: 1056-1063. PubMed: https://pubmed.ncbi.nlm.nih.gov/22192397/
- Pineda M, Reimers TJ, Faulkner LC, Hopwood ML, Seidel Jr, GE. Azoospermia in dogs induced by injection of sclerosing agents into the caudae of the epididymides. Am J Vet Res; 1977; 38: 831-838. PubMed: https://pubmed.ncbi.nlm.nih.gov/560154/
- Immegart HM, Threlfall WR. Evaluation of intratesticular injection of glycerol for nonsurgical sterilization of dogs. Am J Vet Res. 2000; 61: 544-549. PubMed: https://pubmed.ncbi.nlm.nih.gov/10803650/
- Abshenas, J, Molaei MM, Derakhshnfar A, Ghalekhani N. Chemical Sterilization by Intratesticular Injection of Eugenia Caryophyllata Essential Oil in Dog: A Histopathological Study. Iranian J Vet Surg. 2013; 8: 9-16.
- Canpolat I, Karabulut E, Eröksüz Y. Chemical castration of adult and non-adult male dogs with sodium chloride solution. Journal of Agri Vet Sci. 2016; 9: 9-11.
- Pineda M, Dooley M. Surgical and chemical vasectomy in the cat. Am J Vet Res. 1984; 45: 291-300. PubMed: https://pubmed.ncbi.nlm.nih.gov/6711952/
- Jana K, Samanta PK. Clinical evaluation of non-surgical sterilization of male cats with single intra-testicular injection of calcium chloride. BMC Vet Res. 2011; 7: 39. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3152893/
- Fagundes AKF, Oliveira ECS, Tenorio BM, Melo CCS, Nery LTB. et al. Injection of chemical castration agent, zinc gluconate, into the testes of cats results in the impairment of spermatogenesis: A potentially irreversible contraceptive approach for this species. Theriogenology. 2014; 81: 230-236. PubMed: https://pubmed.ncbi.nlm.nih.gov/24238399/
- Neto OA, Gasperin BG, Rovani MT, Ilha GF, Nóbrega Jr JE, et al. Intratesticular hypertonic sodium chloride solution treatment as a method of chemical castration in cattle. Theriogenology. 2014; 82: 1007-1011. PubMed: https://pubmed.ncbi.nlm.nih.gov/25149022/
- Canpolat I, Gur S, Gunay C, Bulut S. An evaluation of the outcome of bull castration by intra-testicular injection of ethanol and calcium chloride. Revue de Méd Vét. 2006; 157: 420.
- Capucille DJ, Poore MH, Rogers GM. Castration in cattle: techniques and animal welfare issues. Compendium, 2002. 24: 66-73.
- Ijaz A, Abalkhail A, Khamas W. Effect of intra testicular injection of formalin on seminiferous tubules in Awassi lambs. Pakistan Vet J. 2000; 20: 129-134.
- Jana K, Samanta PK, Ghosh D. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization of male Black Bengal goats (Capra hircus): a dose-dependent study. Anim Reprod Sci. 2005; 86: 89-108. PubMed: https://pubmed.ncbi.nlm.nih.gov/15721661/
- Mohammed A, James F. Chemical castration by a single bilateral intra-testicular injection of chlorhexidine gluconate and cetrimide in bucks. Sokoto J Vet Sci. 2013; 11: 62-65.
- >Hassan A, Fromsa A. Review on Chemical Sterilization of Male Dogs. Int J Adv Res. 2017; 5: 758-770.
- Pařizek J. The third oliver bird lecture sterilization of the male by cadmium salts. Reproduction. 1960; 1: 294-309.
- Heath E, Arowolo R. The Early Histopathologic Effects of Intratesticular Injection with Hyperosmolar Glycerol, Glucose or NaCI Solutions. Rologia. 1987; 19: 654-661. PubMed: https://pubmed.ncbi.nlm.nih.gov/3434855/
- Fordyce G, Hodge PB, Beaman NJ, Laing AR, Campero C, et al. An evaluation of calf castration by intra-testicular injection of a lactic acid solution [beef cattle]. Aust Vet J.1989; 66 272-276. PubMed: https://pubmed.ncbi.nlm.nih.gov/2684125/
- Alper B, İbrahim C. Chemical sterilization in domestic animals. Research in Agricultural and Veterinary Sciences. 2019; 3: 5-9.
- Wiebe JP, Barr KJ, Buckingham KD. Sustained azoospermia in squirrel monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception. 1989; 39: 447-457.
- NIH, Guide for the care and use of laboratory animals, in National Institute of Health. Bethesda (Md) 7 US Department of Health and Human Services. 2010.
- NRC, Guidelines for care and use of animals in scientific research, in National Research Council. National Academies Press: New Delhi (India) Indian National Science Academy. 2002.
- Petrie A, Watson. Statistics for veterinary and animal science. Balckwell Science Ltd.: Malden, USA.1999.
- Woodall P, Johnstone I. Scrotal width as an index of testicular size in dogs and its relationship to body size. Journal of Small Animal Practice, 2008. 29: 543-547.
- Talukder M. Histopathology Techniques: Tissue Processing and Stainning. 2007: http://www.talukderb.com .
- Fox KA, Diamond B, Sun F, Clavijo A, Sneed L, et al. Testicular lesions and antler abnormalities in Colorado, USA mule deer (Odocoileus hemionus): a possible role for epizootic hemorrhagic disease virus. J Wildlife Dis. 2015; 51: 166-176. PubMed: https://pubmed.ncbi.nlm.nih.gov/25375947
- Freeman C, Coffey D. Sterility in male animals induced by injection of chemical agents into the vas deferens. Ferti Sterility. 1973; 24: 884-890. PubMed: https://pubmed.ncbi.nlm.nih.gov/4742009/
- Hill G, Neville JW, Richardson K. Castration Method and Progesterone-Estradiol Implant Effects on Growth Rate of Suckling Calves. J Dairy Sci. 1985; 68: 3059-3061.
- Igdoura S, Wiebe J. Suppression of Spermatogenesis by Low Level of Glycerol Treatment. J Androl. 1994; 15: 234-243.
- Oliveira E, Moura M, Silva JV. Intratesticular injection of a zinc-based solution as a contraceptive for dogs. Theriogenology. 2007; 68: 137-145.
- Russell L, Saxena N, Weber J. Intratesticular injection as a method to assess the potential toxicity of various agents and to study mechanisms of normal spermatogenesis. Gamete Res. 1987; 17: 43-56. PubMed: https://pubmed.ncbi.nlm.nih.gov/2906900/
- Karmakar SN, Das SK. Chemosterilization induced by intratesticular injection of calcium chloride (CaCl2)-a tool for population control. Int J Pharmaceut Che Biol Sci. 2017; 7: 25-35.
- Cavalieri J. Chemical sterilisation of animals: A review of the use of zinc-and CaCl2 based solutions in male and female animals and factors likely to improve responses to treatment. Anim Reprod Sci. 2017; 181: 1-8. PubMed: https://pubmed.ncbi.nlm.nih.gov/28366279/
- McGinnis K, Wangi K, Genegy M. Alterations of extra-cellular calcium elicits selective modes of cell death and protease activation in SH-SY5Y human neuroblastoma cells. J Neurochem. 1999; 72: 1853– 1863. PubMed: https://pubmed.ncbi.nlm.nih.gov/10217261
- Oliveira EC, Fagundes AKF, Meloc CCS, Nery LTB, Rêvoredo RG, et al. Intratesticular injection of a zinc-based solution for contraception of domestic cats: a randomized clinical trial of efficacy and safety. Vet J. 2013; 197: 307-310.
- Doerksen T, Bonoit G, Trasler J. Deoxyribonucleic acid hypomethylation of male germ cells by mitotic and meiotic exposure to 5-azacytidine is associated with altered testicular histology. Endocrinology. 2000; 141: 3235-3244. PubMed: https://pubmed.ncbi.nlm.nih.gov/10965894/
- Emir L, Dadali M, Sunay M, Erol D, Caydere M, et al. Chemical castration with intratesticular injection of 20% hypertonic saline: A minimally invasive method. Urol Oncol. 2008; 26: 392-396. PubMed: https://pubmed.ncbi.nlm.nih.gov/18367099/
- Tillbrook A, Clarke I. Negative feedback regulation of the secretion and actions of gonadotrophin releasing hormone in males. Biol Reprod. 2001. 64: 735– 742. PubMed: https://pubmed.ncbi.nlm.nih.gov/11207186/
- Xu C, Lu MG, Feng JS, Guo QS, Wang YF. Germ cell apoptosis induced by Ureaplasma urealytium infection. Asian J Androl. 2001; 3: 199-204. PubMed: https://pubmed.ncbi.nlm.nih.gov/11561190/
- Sharpe R, Maddocks S, Millar M, Kerr JB, Saunders PT, et al. Testosterone and Spermatogenesis Identification of Stage‐Specific, Androgen‐Regulated Proteins Secreted by Adult Rat Seminiferous Tubules. J Androl. 1992; 13: 172-184. PubMed: https://pubmed.ncbi.nlm.nih.gov/1317835/
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2, 4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001; 64: 1500-1508. PubMed: https://pubmed.ncbi.nlm.nih.gov/11319158/
Figures:

Figure 1

Figure 2
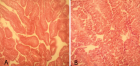
Figure 3
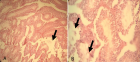
Figure 4
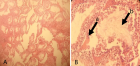
Figure 5
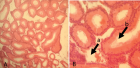
Figure 6
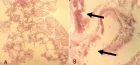
Figure 7
Similar Articles
-
Evaluation of Single Bilateral Intratesticular Injection of Cetrimide for Nonsurgical Sterilization of Adult Male Albino MiceHaben Fesseha*,Guesh Negash. Evaluation of Single Bilateral Intratesticular Injection of Cetrimide for Nonsurgical Sterilization of Adult Male Albino Mice. . 2020 doi: 10.29328/journal.ivs.1001023; 4: 025-034
Recently Viewed
-
A Review on Heavy Metals in Ecosystems, Their Sources, Roles, and Impact on Plant LifeHumaira Aslam, Ali Umar, Misbah Ullah Khan*, Shehla Honey, Aman Ullah, Muhammad Ahsan Ashraf, Ghulam Ayesha, Nazia Nusrat, M Jamil, Shahid Khan, Adeel Abid. A Review on Heavy Metals in Ecosystems, Their Sources, Roles, and Impact on Plant Life. J Genet Med Gene Ther. 2024: doi: 10.29328/journal.jgmgt.1001012; 7: 020-034
-
Germline BRCA1 Mutation inSquamous Cell Carcinoma of Oesophagus: Driver versus Passenger MutationAmrit Kaur Kaler*, Shraddha Manoj Upadhyay, Nandini Shyamali Bora, Ankita Nikam, Kavya P, Nivetha Athikeri, Dattatray B Solanki, Imran Shaikh, Rajesh Mistry. Germline BRCA1 Mutation inSquamous Cell Carcinoma of Oesophagus: Driver versus Passenger Mutation. J Genet Med Gene Ther. 2024: doi: 10.29328/journal.jgmgt.1001011; 7: 015-019
-
A Critical Review on Some Recent Developments in Comparison of Biological SequencesDK Bhattacharya*. A Critical Review on Some Recent Developments in Comparison of Biological Sequences. J Genet Med Gene Ther. 2024: doi: 10.29328/journal.jgmgt.1001010; 7: 008-014
-
Management and Therapeutic Strategies for Spinal Muscular AtrophySheena P Kochumon, Cherupally Krishnan Krishnan Nair*. Management and Therapeutic Strategies for Spinal Muscular Atrophy. J Genet Med Gene Ther. 2024: doi: 10.29328/journal.jgmgt.1001009; 7: 001-007
-
Pituitary gland metastasis from breast cancer: case reportMohamed Almadhoni,Mohamed Ali Baggas*. Pituitary gland metastasis from breast cancer: case report. Arch Cancer Sci Ther. 2022: doi: 10.29328/journal.acst.1001025; 6: 001-003
Most Viewed
-
Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trialSathit Niramitmahapanya*,Preeyapat Chattieng,Tiersidh Nasomphan,Korbtham Sathirakul. Effects of dietary supplementation on progression to type 2 diabetes in subjects with prediabetes: a single center randomized double-blind placebo-controlled trial. Ann Clin Endocrinol Metabol. 2023 doi: 10.29328/journal.acem.1001026; 7: 00-007
-
Physical Performance in the Overweight/Obesity Children Evaluation and RehabilitationCristina Popescu, Mircea-Sebastian Șerbănescu, Gigi Calin*, Magdalena Rodica Trăistaru. Physical Performance in the Overweight/Obesity Children Evaluation and Rehabilitation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001030; 8: 004-012
-
Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging PresentationKarthik Baburaj*, Priya Thottiyil Nair, Abeed Hussain, Vimal MV. Hypercalcaemic Crisis Associated with Hyperthyroidism: A Rare and Challenging Presentation. Ann Clin Endocrinol Metabol. 2024 doi: 10.29328/journal.acem.1001029; 8: 001-003
-
Exceptional cancer responders: A zone-to-goDaniel Gandia,Cecilia Suárez*. Exceptional cancer responders: A zone-to-go. Arch Cancer Sci Ther. 2023 doi: 10.29328/journal.acst.1001033; 7: 001-002
-
The benefits of biochemical bone markersSek Aksaranugraha*. The benefits of biochemical bone markers. Int J Bone Marrow Res. 2020 doi: 10.29328/journal.ijbmr.1001013; 3: 027-031

If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."